Search
- Page Path
- HOME > Search
- [Korean]
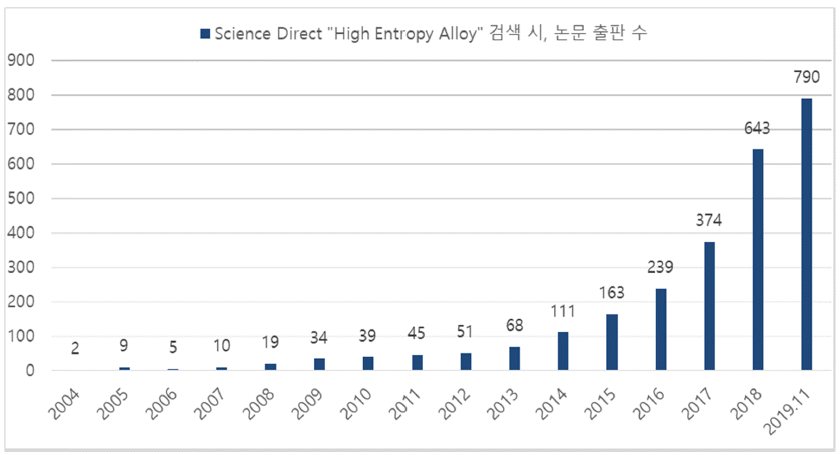
- Research Trends of High-entropy Alloys
- Pureunsol Park, Ho Joon Lee, Youngjun Jo, Bonseung Gu, Won June Choi, Jongmin Byun
- J Korean Powder Metall Inst. 2019;26(6):515-527. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.515
- 5,690 View
- 44 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF High-entropy alloys (HEAs) are generally defined as solid solutions containing at least 5 constituent elements with concentrations between 5 and 35 atomic percent without the formation of intermetallic compounds. Currently, HEAs receive great attention as promising candidate materials for extreme environments due to their potentially desirable properties that result from their unique structural properties. In this review paper, we aim to introduce HEAs and explain their properties and related research by classifying them into three main categories, namely, mechanical properties, thermal properties, and electrochemical properties. Due to the high demand for structural materials in extreme environments, the mechanical properties of HEAs including strength, hardness, ductility, fatigue, and wear resistance are mainly described. Thermal and electrochemical properties, essential for the application of these alloys as structural materials, are also described.
-
Citations
Citations to this article as recorded by- Composites of equiatomic Y, La, Ce, Nd, and Gd rare earth oxides: Chemical-shift effects and valence spectra
Jungsu Bin, Hyunbae Gee, Taesung Park, UiJun Go, Jeoung Han Kim, Youn-Seoung Lee
Current Applied Physics.2024; 59: 85. CrossRef - Sintering Behavior and Mechanical Property of Transition Metal Carbide-Based Cermets by Spark Plasma Sintering
Jeong-Han Lee, Hyun-Kuk Park, Sung-Kil Hong
Korean Journal of Materials Research.2022; 32(1): 44. CrossRef
- Composites of equiatomic Y, La, Ce, Nd, and Gd rare earth oxides: Chemical-shift effects and valence spectra
- [Korean]
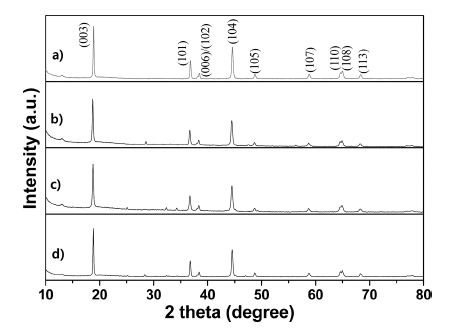
- Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
- Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2019;26(1):49-57. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.49
- 2,009 View
- 22 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Layered LiNi0.83Co0.11Mn0.06O2 cathode materials single- and dual-doped by the rare-earth elements Ce and Nd are successfully fabricated by using a coprecipitation-assisted solid-phase method. For comparison purposes, nondoping pristine LiNi0.83Co0.11Mn0.06O2 cathode material is also prepared using the same method. The crystal structure, morphology, and electrochemical performances are characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometer (EDS) mapping, and electrochemical techniques. The XRD data demonstrates that all prepared samples maintain a typical α-NaFeO2-layered structure with the
R-3m -
Citations
Citations to this article as recorded by- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
Heewon Choi, Nam-gyu Lim, Seong Jun Lee, Jungsoo Park
Journal of Mechanical Science and Technology.2021; 35(6): 2697. CrossRef - Synthesis of CeVO4-V2O5 nanowires by cation-exchange method for high-performance lithium-ion battery electrode
Xueliu Xu, Shiying Chang, Taofang Zeng, Yidan Luo, Dong Fang, Ming Xie, Jianhong Yi
Journal of Alloys and Compounds.2021; 887: 161237. CrossRef
- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
- [Korean]
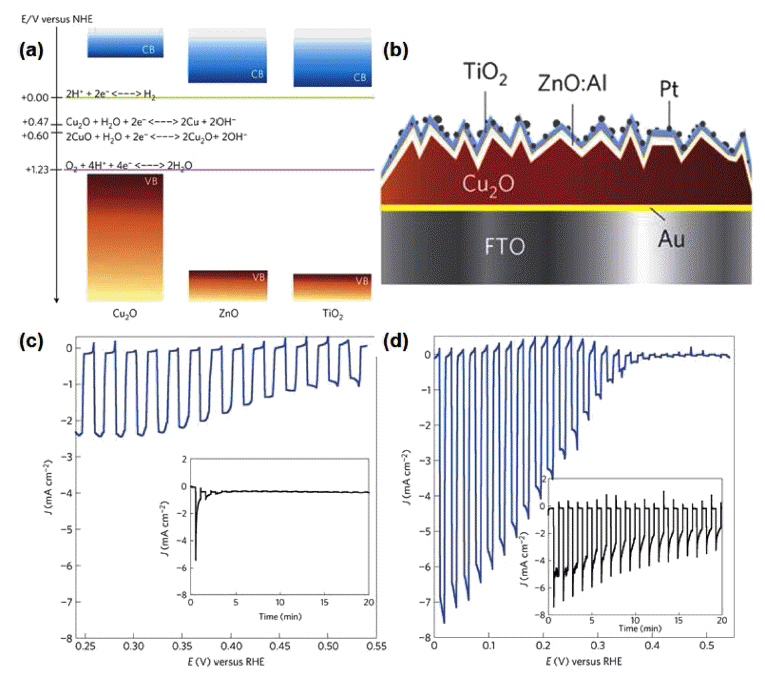
- Recent Developments in H2 Production Photoelectrochemical Electrode Materials by Atomic Layer Deposition
- Jeong Hwan Han
- J Korean Powder Metall Inst. 2018;25(1):60-68. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.60
- 822 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The design and fabrication of photoelectrochemical (PEC) electrodes for efficient water splitting is important for developing a sustainable hydrogen evolution system. Among various development approaches for PEC electrodes, the chemical vapor deposition method of atomic layer deposition (ALD), based on self-limiting surface reactions, has attracted attention because it allows precise thickness and composition control as well as conformal coating on various substrates. In this study, recent research progress in improving PEC performance using ALD coating methods is discussed, including 3D and heterojunction-structured PEC electrodes, ALD coatings of noble metals, and the use of sulfide materials as co-catalysts. The enhanced long-term stability of PEC cells by ALD-deposited protecting layers is also reviewed. ALD provides multiple routes to develop improved hydrogen evolution PEC cells.
-
Citations
Citations to this article as recorded by- Improved Interface and Electrical Properties by Inserting an Ultrathin SiO2 Buffer Layer in the Al2O3/Si Heterojunction
Doyeon Kim, Jae‐Young Choi, Sang Wook Ryu, Woo‐Byoung Kim
Advanced Functional Materials.2019;[Epub] CrossRef
- Improved Interface and Electrical Properties by Inserting an Ultrathin SiO2 Buffer Layer in the Al2O3/Si Heterojunction
- [Korean]
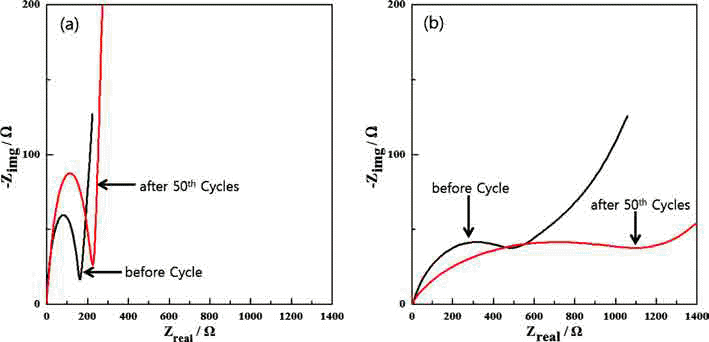
- Fabrication of Fe3O4/Fe/Graphene nanocomposite powder by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ji-Seub Choi, Hoi-Jin Lee, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2017;24(4):308-314. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.308
- 1,022 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Fe3O4/Fe/graphene nanocomposite powder is synthesized by electrical wire explosion of Fe wire and dispersed graphene in deionized water at room temperature. The structural and electrochemical characteristics of the powder are characterized by the field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, field-emission transmission electron microscopy, cyclic voltammetry, and galvanometric discharge-charge method. For comparison, Fe3O4/Fe nanocomposites are fabricated under the same conditions. The Fe3O4/Fe nanocomposite particles, around 15-30 nm in size, are highly encapsulated in a graphene matrix. The Fe3O4/Fe/graphene nanocomposite powder exhibits a high initial charge specific capacity of 878 mA/g and a high capacity retention of 91% (798 mA/g) after 50 cycles. The good electrochemical performance of the Fe3O4/Fe/graphene nanocomposite powder is clearly established by comparison of the results with those obtained for Fe3O4/Fe nanocomposite powder and is attributed to alleviation of volume change, good distribution of electrode active materials, and improved electrical conductivity upon the addition of graphene.
-
Citations
Citations to this article as recorded by- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
Kyunbae Lee, Joonsik Lee, Byung Mun Jung, Byeongjin Park, Taehoon Kim, Sang Bok Lee
Applied Surface Science.2019; 478: 733. CrossRef
- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
- [Korean]
- Effects of Porous Microstructure on the Electrochemical Properties of Si-Ge-Al Base Anode Materials for Li-ion Rechargeable Batteries
- Chung Rae Cho, Myeong Geun Kim, Keun Yong Sohn, Won-Wook Park
- J Korean Powder Metall Inst. 2017;24(1):24-28. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.24
- 654 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Silicon alloys are considered promising anode active materials to replace Li-ion batteries by graphite powder, because they have a relatively high capacity of up to 4200 mAh/g, and are environmentally friendly and inexpensive ECO-materials. However, its poor charge/discharge properties, induced by cracking during cycles, constitute their most serious problem as anode electrode. In order to solve these problems, Si-Ge-Al alloys with porous structure are designed as anode alloy powders, to improve cycling stability. The alloys are melt-spun to obtain the rapidly solidified ribbons, and then ball-milled to make fine powders. The powders are etched using 1 M HCl solution, which gives the powders a porous structure by removing the element Al. Subsequently, in this study, the microstructures and the characteristics of the etched powders are evaluated for application as anode materials. As a result, the etched porous powder shows better electrochemical properties than as-milled Si-Ge-Al powder.
- [Korean]
- Fabrication of Carbon-coated Tin Nano-powders by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ju-Suck Song, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2016;23(4):317-324. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.317
- 999 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Tin is one of the most promising anode materials for next-generation lithium-ion batteries with a high energy density. However, the commercialization of tin-based anodes is still hindered due to the large volume change (over 260%) upon lithiation/delithiation cycling. To solve the problem, many efforts have been focused on enhancing structural stability of tin particles in electrodes. In this work, we synthesize tin nano-powders with an amorphous carbon layer on the surface and surroundings of the powder by electrical wire explosion in alcohol-based liquid media at room temperature. The morphology and microstructures of the powders are characterized by scanning electron microscopy, Xray diffraction, Raman spectroscopy, and transmission electron microscopy. The electrochemical properties of the powder for use as an anode material for lithium-ion battery are evaluated by cyclic voltammetry and a galvanometric dischargecharge method. It is shown that the carbon-coated tin nano-powders prepared in hexanol media exhibit a high initial charge specific capacity of 902 mAh/g and a high capacity retention of 89% after 50 cycles.
-
Citations
Citations to this article as recorded by- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
Ji-Seub Choi, Yeon-Ju Lee, Hoi-Jin Lee, Gyu-Bong Cho, Jai-Won Byeon, Hyo-Jun Ahn, Ki-Won Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Journal of Alloys and Compounds.2020; 843: 155892. CrossRef - Fabrication of multilayer graphene-encapsulated Sn/SnO2 nanocomposite as an anode material for lithium-ion batteries and its electrochemical properties
Ju-Seok Song, Gyu-Bong Cho, Ki-Won Kim, Hyo-Jun Ahn, Hye-Sung Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Applied Surface Science.2019; 481: 736. CrossRef
- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
- [English]
- Using Carboxylmethylated Cellulose as Water-Borne Binder to Enhance the Electrochemical Properties of Li4Ti5O12-Based Anodes
- Lili Liu, Chongling Cheng, Hongjiang Liu, Liyi Shi, Dayang Wang
- J Korean Powder Metall Inst. 2015;22(5):315-320. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.315
- 1,264 View
- 8 Download
-
 Abstract
Abstract
 PDF
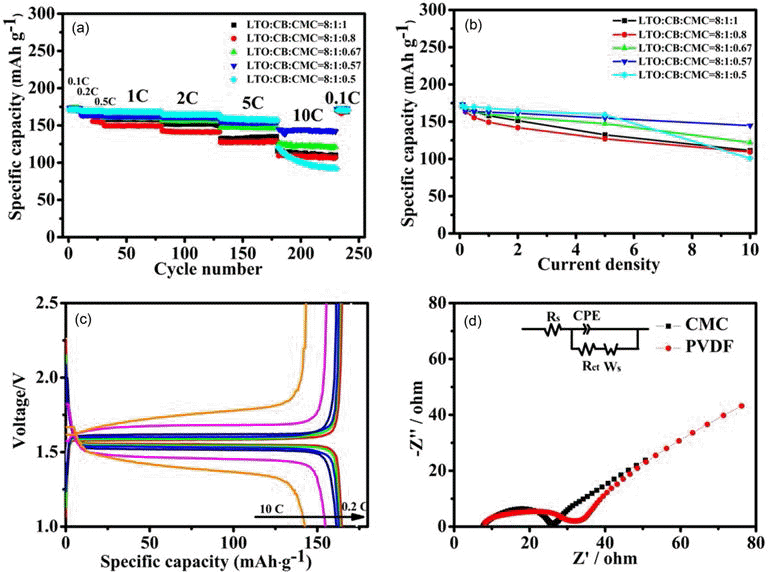
PDF The present work reports a systematic study of using carboxymethylated cellulose (CMC) as water-borne binder to produce Li4Ti5O12-based anodes for manufacture of high rate performance lithium ion batteries. When the LTO-to-CB-to-CMC mass ratio is carefully optimized to be 8:1:0.57, the special capacity of the resulting electrodes is 144 mAh·g−1 at 10 C and their capacity retention was 97.7% after 1000 cycles at 1 C and 98.5% after 500 cycles at 5 C, respectively. This rate performance is comparable or even better than that of the electrolytes produced using conventional, organic, polyvinylidene fluoride binder.
- [Korean]
- Fabrication of LiNiO2 using NiSO4 Recovered from NCM (Li[Ni,Co,Mn]O2) Secondary Battery Scraps and Its Electrochemical Properties
- Yong-Gyu Kwag, Mi-So Kim, Yoo-Young Kim, Im-Sic Choi, Dong-Kyu Park, In-Sup Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2014;21(4):286-293. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.286

- 912 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The electrochemical properties of cells assembled with the LiNiO2 (LNO) recycled from cathode materials of waste lithium secondary batteries (Li[Ni,Co,Mn]O2), were evaluated in this study. The leaching, neutralization and solvent extraction process were applied to produce high-purity NiSO4 solution from waste lithium secondary batteries. High-purity NiO powder was then fabricated by the heat-treatment and mixing of the NiSO4 solution and H2C2O4. Finally, LiNiO2 as a cathode material for lithium ion secondary batteries was synthesized by heat treatment and mixing of the NiO and Li2CO3 powders. We assembled the cells using the LiNiO2 powders and evaluated the electrochemical properties. Subsequently, we evaluated the recycling possibility of the cathode materials for waste lithium secondary battery using the processes applied in this work.
TOP
 KPMI
KPMI


 First
First Prev
Prev


