Search
- Page Path
- HOME > Search
- [Korean]
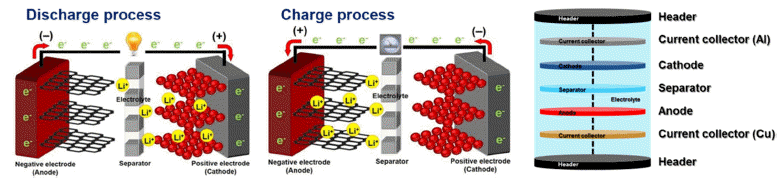
- Research Trends of Cathode Materials for Lithium-Ion Batteries used in Electric Vehicles
- Dong-Yo Shin, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2019;26(1):58-69. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.58
- 1,017 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF High performance lithium-ion batteries (LIBs) have attracted considerable attention as essential energy sources for high-technology electrical devices such as electrical vehicles, unmanned drones, uninterruptible power supply, and artificial intelligence robots because of their high energy density (150-250 Wh/kg), long lifetime (> 500 cycles), low toxicity, and low memory effects. Of the high-performance LIB components, cathode materials have a significant effect on the capacity, lifetime, energy density, power density, and operating conditions of high-performance LIBs. This is because cathode materials have limitations with respect to a lower specific capacity and cycling stability as compared to anode materials. In addition, cathode materials present difficulties when used with LIBs in electric vehicles because of their poor rate performance. Therefore, this study summarizes the structural and electrochemical properties of cathode materials for LIBs used in electric vehicles. In addition, we consider unique strategies to improve their structural and electrochemical properties.
-
Citations
Citations to this article as recorded by- Estimation of Representative Mechanical Property of Porous Electrode for Secondary Batteries with Homogenization Method
Changmin Pyo, Jaewoong Kim
Journal of the Korean Society of Manufacturing Process Engineers.2022; 21(9): 85. CrossRef
- Estimation of Representative Mechanical Property of Porous Electrode for Secondary Batteries with Homogenization Method
- [Korean]
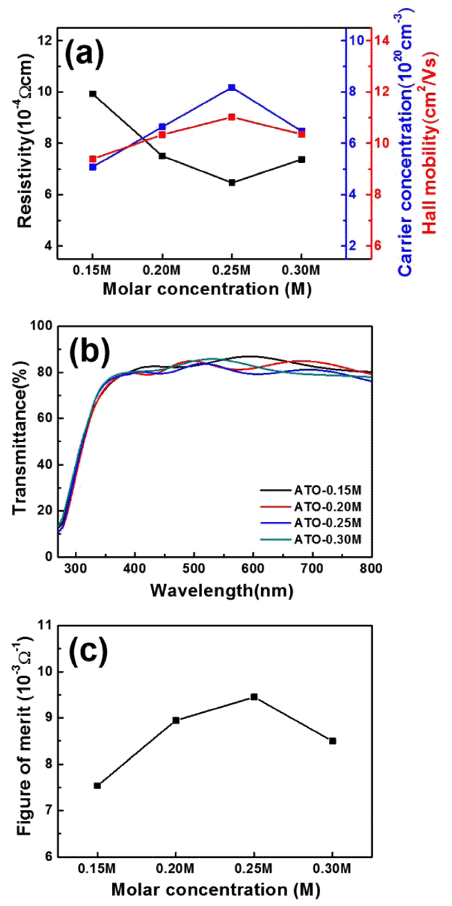
- Fabrication of compact surface structure by molar concentration on Sb-doped SnO2 transparent conducting films
- Ju-Won Bae, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2018;25(1):54-59. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.54
- 732 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Sb-doped SnO2 (ATO) transparent conducting films are fabricated using horizontal ultrasonic spray pyrolysis deposition (HUSPD) to form uniform and compact film structures with homogeneously supplied precursor solution. To optimize the molar concentration and transparent conducting performance of the ATO films using HUSPD, we use precursor solutions of 0.15, 0.20, 0.25, and 0.30 M. As the molar concentration increases, the resultant ATO films exhibit more compact surface structures because of the larger crystallite sizes and higher ATO crystallinity because of the greater thickness from the accelerated growth of ATO. Thus, the ATO films prepared at 0.25 M have the best transparent conducting performance (12.60±0.21 Ω/□ sheet resistance and 80.83% optical transmittance) and the highest figure-of-merit value (9.44±0.17 × 10-3 Ω-1). The improvement in transparent conducting performance is attributed to the enhanced carrier concentration by the improved ATO crystallinity and Hall mobility with the compact surface structure and preferred (211) orientation, ascribed to the accelerated growth of ATO at the optimized molar concentration. Therefore, ATO films fabricated using HUSPD are transparent conducting film candidates for optoelectronic devices.
- [Korean]
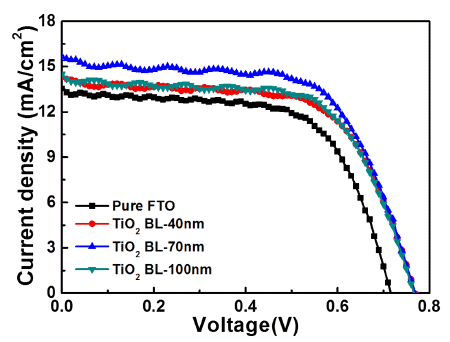
- Fabrication of Uniform TiO2 Blocking Layers for Prevention of Electron Recombination in Dye-Sensitized Solar Cells
- Ju-won Bae, Bon-Ryul Koo, Tae-Kuen Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2018;25(1):1-6. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.1
- 751 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Uniform TiO2 blocking layers (BLs) are fabricated using ultrasonic spray pyrolysis deposition (USPD) method. To improve the photovoltaic performance of dye-sensitized solar cells (DSSCs), the BL thickness is controlled by using USPD times of 0, 20, 60, and 100 min, creating TiO2 BLs of 0, 40, 70, and 100 nm, respectively, in average thickness on fluorine-doped tin oxide (FTO) glass. Compared to the other samples, the DSSC containing the uniform TiO2 BL of 70 nm in thickness shows a superior power conversion efficiency of 7.58±0.20% because of the suppression of electron recombination by the effect of the optimized thickness. The performance improvement is mainly attributed to the increased open-circuit voltage (0.77±0.02 V) achieved by the increased Fermi energy levels of the working electrodes and the improved short-circuit current density (15.67±0.43 mA/cm2) by efficient electron transfer pathways. Therefore, optimized TiO2 BLs fabricated by USPD may allow performance improvements in DSSCs.
-
Citations
Citations to this article as recorded by- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
Seung Hwan Ji, Hyunsu Park, Doyeon Kim, Do Hyung Han, Hye Won Yun, Woo-Byoung Kim
Korean Journal of Materials Research.2019; 29(3): 183. CrossRef - Surface tailoring of zinc electrodes for energy storage devices with high-energy densities and long cycle life
Geon-Hyoung An, SeungNam Cha, Jung Inn Sohn
Applied Surface Science.2019; 467-468: 1157. CrossRef - Crystallinity Control Effects on Vanadium Oxide Films for Enhanced Electrochromic Performances
Kue-Ho Kim, Ju-Won Bae, Tae-Kuen Lee, Hyo-Jin Ahn
Korean Journal of Materials Research.2019; 29(6): 385. CrossRef
- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
- [Korean]
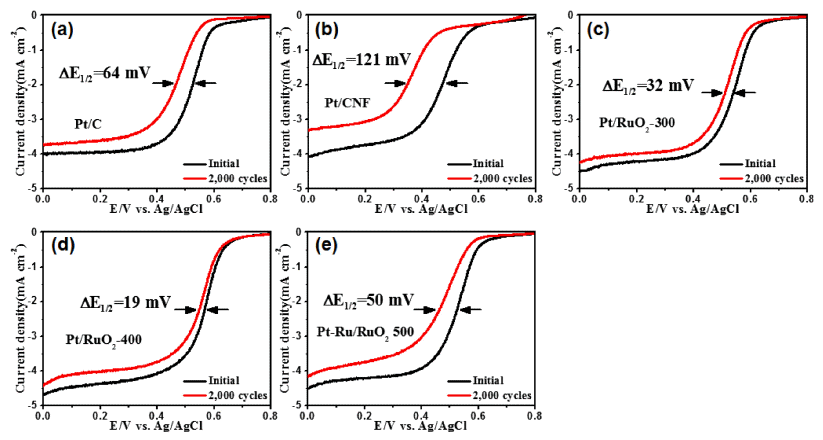
- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
- Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2017;24(2):96-101. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.96
- 911 View
- 3 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Well-dispersed platinum catalysts on ruthenium oxide nanofiber supports are fabricated using electrospinning, post-calcination, and reduction methods. To obtain the well-dispersed platinum catalysts, the surface of the nanofiber supports is modified using post-calcination. The structures, morphologies, crystal structures, chemical bonding energies, and electrochemical performance of the catalysts are investigated. The optimized catalysts show well-dispersed platinum nanoparticles (1-2 nm) on the nanofiber supports as well as a uniform network structure. In particular, the well-dispersed platinum catalysts on the ruthenium oxide nanofiber supports display excellent catalytic activity for oxygen reduction reactions with a half-wave potential (E1/2) of 0.57 V and outstanding long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 19 mV. The enhanced electrochemical performance for oxygen reduction reactions results from the well-dispersed platinum catalysts and unique nanofiber supports.
-
Citations
Citations to this article as recorded by- Fabrication of Porous Electrodes for Zinc-Ion Supercapacitors with Improved Energy Storage Performance
Geon-Hyoung An
Korean Journal of Materials Research.2019; 29(8): 505. CrossRef - Surface tailoring of zinc electrodes for energy storage devices with high-energy densities and long cycle life
Geon-Hyoung An, SeungNam Cha, Jung Inn Sohn
Applied Surface Science.2019; 467-468: 1157. CrossRef - Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef
- Fabrication of Porous Electrodes for Zinc-Ion Supercapacitors with Improved Energy Storage Performance
- [Korean]
- Research Trends in Powder Materials for Solution-based Transparent Conducting Electrode
- Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2017;24(2):153-163. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.153
- 815 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
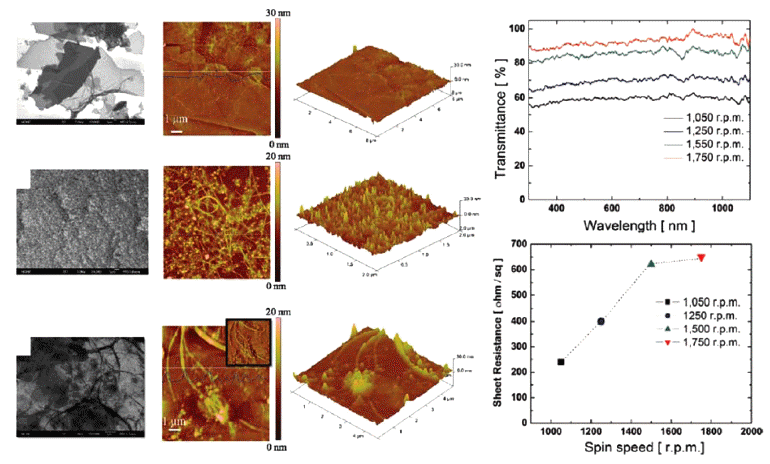
PDF Transparent conducting electrodes (TCEs) are attracting considerable attention as an important component for emerging optoelectronic applications such as liquid crystal displays, touch panels, and solar cells owing to their attractive combination of low resistivity (< 10-3 Ω cm) and high transparency (>80%) in the visible region. The solutionbased process has unique properties of an easy fabrication procedure, scalability, and low cost compared to the conventional vacuum-based process and may prove to be a useful process for fabricating TCEs for future optoelectronic applications demanding large scale and flexibility. In this paper, we focus on the introduction of a solution-based process for TCEs. In addition, we consider the powder materials used to fabricate solution-based TCEs and strategies to improve their transparent conducting properties.
-
Citations
Citations to this article as recorded by- Electrically conductive and anti-corrosive coating on copper foil assisted by polymer-nanocomposites embedded with graphene
Han Kim, Hyemin Lee, Hyo-Ryoung Lim, Hong-Baek Cho, Yong-Ho Choa
Applied Surface Science.2019; 476: 123. CrossRef
- Electrically conductive and anti-corrosive coating on copper foil assisted by polymer-nanocomposites embedded with graphene
- [Korean]
- Synthesis of Nitrogen-doped Carbon Nanofibers for Oxygen Reduction Reaction
- Geon-Hyoung An, Eun-Hwan Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(6):420-425. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.420

- 637 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF N-doped carbon nanofibers as catalysts for oxygen-reduction reactions are synthesized using electrospinning and carbonization. Their morphologies, structures, chemical bonding states, and electrochemical performance are characterized. The optimized N-doped carbon nanofibers exhibit graphitization of carbon nanofibers and an increased nitrogen doping as well as a uniform network structure. In particular, the optimized N-doped carbon nanofibers show outstanding catalytic activity for oxygen-reduction reactions, such as a half-wave potential (E1/2) of 0.43 V, kinetic limiting current density of 6.2 mA cm-2, electron reduction pathways (n = 3.1), and excellent long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 13 mV. The improvement in the electrochemical performance results from the synergistic effect of the graphitization of carbon nanofibers and the increased amount of nitrogen doping.
- [Korean]
- Spindle-shaped Fe2O3 Nanoparticle Coated Carbon Nanofiber Composites for Low-cost Dye-sensitized Solar Cells
- Dong-Hyeun Oh, HyeLan An, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(2):95-101. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.95
- 1,036 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
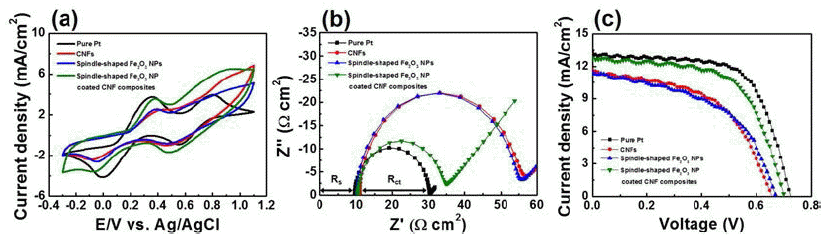
PDF Carbon nanofiber (CNF) composites coated with spindle-shaped Fe2O3 nanoparticles (NPs) are fabricated by a combination of an electrospinning method and a hydrothermal method, and their morphological, structural, and chemical properties are measured by field-emission scanning electron microscopy, transmission electron microscopy, Xray diffraction, and X-ray photoelectron spectroscopy. For comparison, CNFs and spindle-shaped Fe2O3 NPs are prepared by either an electrospinning method or a hydrothermal method, respectively. Dye-sensitized solar cells (DSSCs) fabricated with the composites exhibit enhanced open circuit voltage (0.70 V), short-circuit current density (12.82 mA/cm2), fill factor (61.30%), and power conversion efficiency (5.52%) compared to those of the CNFs (0.66 V, 11.61 mA/cm2, 51.96%, and 3.97%) and spindle-shaped Fe2O3 NPs (0.67 V, 11.45 mA/cm2, 50.17%, and 3.86%). This performance improvement can be attributed to a synergistic effect of a superb catalytic reaction of spindle-shaped Fe2O3 NPs and efficient charge transfer relative to the one-dimensional nanostructure of the CNFs. Therefore, spindle-shaped Fe2O3-NPcoated CNF composites may be proposed as a potential alternative material for low-cost counter electrodes in DSSCs.
-
Citations
Citations to this article as recorded by- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
Dong-Hyeun Oh, Bon-Ryul Koo, Yu-Jin Lee, HyeLan An, Hyo-Jin Ahn
Korean Journal of Materials Research.2016; 26(11): 649. CrossRef
- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
- Synthesis of Perforated Polygonal Cobalt Oxides using a Carbon Nanofiber Template
- Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(5):350-355. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.350
- 825 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
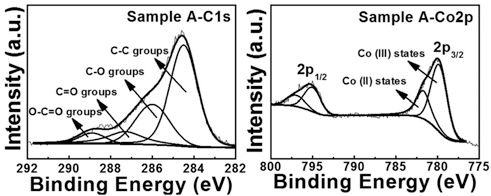
PDF Perforated polygonal cobalt oxide (CO3O4) is synthesized using electrospinning and a hydrothermal method followed by the removal of a carbon nanofiber (CNF) template. To investigate their formation mechanism, thermogravimetric analysis, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and Xray photoelectron spectroscopy are examined. To obtain the optimum condition of perforated polygonal CO3O4, we prepare three different weight ratios of the Co precursor and the CNF template: sample A (Co precursor:CNF template- 10:1), sample B (Co precursor:CNF template-3.2:1), and sample C (Co precursor:CNF template-2:1). Among them, sample A exhibits the perforated polygonal CO3O4 with a thin carbon layer (5.7-6.2 nm) owing to the removal of CNF template. However, sample B and sample C synthesized perforated round CO3O4 and destroyed CO3O4 powders, respectively, due to a decreased amount of Co precursor. The increased amount of the CNF template prevents the formation of polygonal CO3O4. For sample A, the optimized weight ratio of the Co precursor and CNF template may be related to the successful formation of perforated polygonal CO3O4. Thus, perforated polygonal CO3O4 can be applied to electrode materials of energy storage devices such as lithium ion batteries, supercapacitors, and fuel cells.
-
Citations
Citations to this article as recorded by- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef - Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef
- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
- [Korean]
- Improvement of Triboelectric Efficiency using SnO2 Friction Layer for Triboelectric Generator
- No Ho Lee, Jae Rok Shin, Ji Een Yoo, Dong Hun You, Bon-Ryul Koo, Sung Woo Lee, Hyo-Jin Ahn, Byung Joon Choi
- J Korean Powder Metall Inst. 2015;22(5):321-325. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.321
- 1,007 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
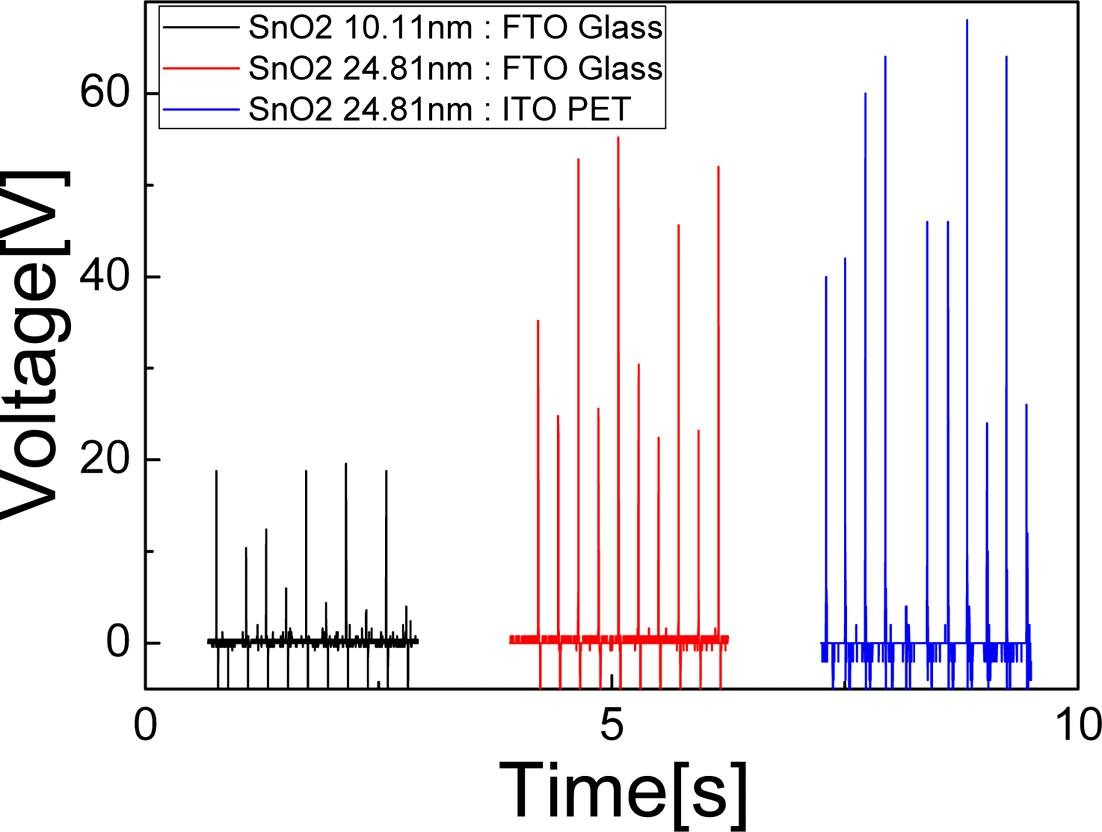
PDF The triboelectric property of a material is important to improve an efficiency of triboelectric generator (TEG) in energy harvesting from an ambient energy. In this study, we have studied the TEG property of a semiconducting SnO2 which has yet to be explored so far. As a counter triboelectric material, PET and glass are used. Vertical contact mode is utilized to evaluate the TEG efficiency. SnO2 thin film is deposited by atomic layer deposition on bare Si wafer for various thicknesses from 5.2 nm to 34.6 nm, where the TEG output is increased from 13.9V to 73.5V. Triboelectric series are determined by comparing the polarity of output voltage of 2 samples among SnO2, PET, and glass. In conclusion, SnO2, as an intrinsic n-type material, has the most strong tendency to be positive side to lose the electron and PET has the most strong tendency to be negative side to get the electron, and glass to be between them. Therefore, the SnO2-PET combination shows the highest TEG efficiency.
-
Citations
Citations to this article as recorded by- Triboelectric charge generation by semiconducting SnO2 film grown by atomic layer deposition
No Ho Lee, Seong Yu Yoon, Dong Ha Kim, Seong Keun Kim, Byung Joon Choi
Electronic Materials Letters.2017; 13(4): 318. CrossRef
- Triboelectric charge generation by semiconducting SnO2 film grown by atomic layer deposition
- [Korean]
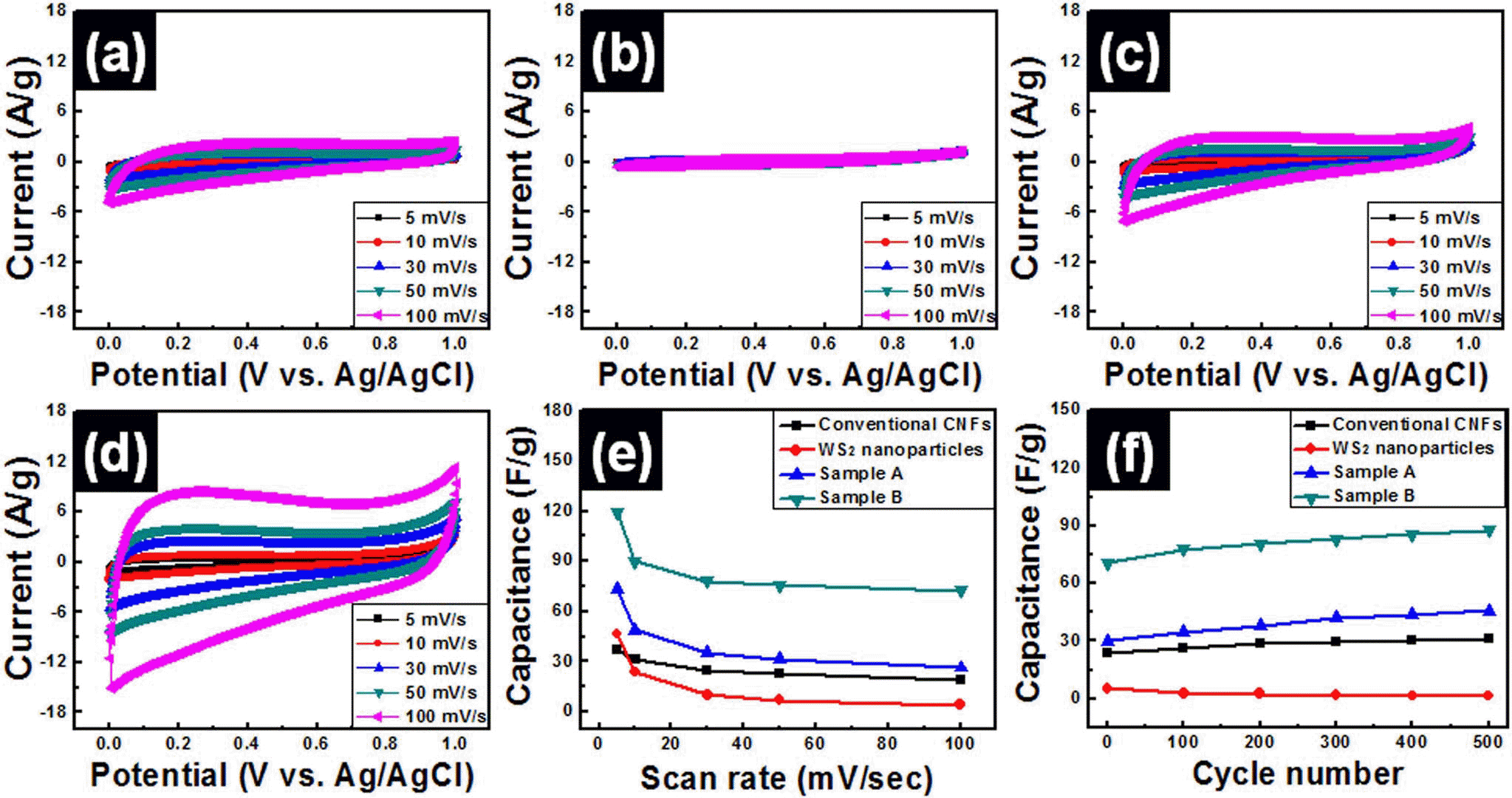
- Fabrication of WS2-W-WC Embedded Carbon Nanofiber Composites for Supercapacitors
- Yu-Jin Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(2):116-121. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.116
- 835 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF WS2-W-WC embedded carbon nanofiber composites were fabricated by using electrospinning method for use in high-performance supercapacitors. In order to obtain optimum electrochemical properties for supercapacitors, WS2 nanoparticles were used as precursors and the amounts of WS2 precursors were controlled to 4 wt% (sample A) and 8 wt% (sample B). The morphological, structural, and chemical properties of all samples were investigated by means of field emission photoelectron spectroscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. These results demonstrated that the embedded phases of samples A and B were changed from WS2 to WS2-W-WC through carbothermal reaction during carbonization process. In particular, sample B presented high specific capacitance (~119.7 F/g at 5 mV/s), good high-rate capacitance (~60.5%), and superb cycleability. The enhanced electrochemical properties of sample B were explained by the synergistic effect of the using 1-D structure supports, increase of specific surface area, and improved conductivity from formation of W and WC phases.
-
Citations
Citations to this article as recorded by- WS2 Nanoparticles Embedded in Carbon Nanofibers for a Pseudocapacitor
Ki-Wook Sung, Jung Soo Lee, Tae-Kum Lee, Hyo-Jin Ahn
Korean Journal of Materials Research.2021; 31(8): 458. CrossRef
- WS2 Nanoparticles Embedded in Carbon Nanofibers for a Pseudocapacitor
- [Korean]
- Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
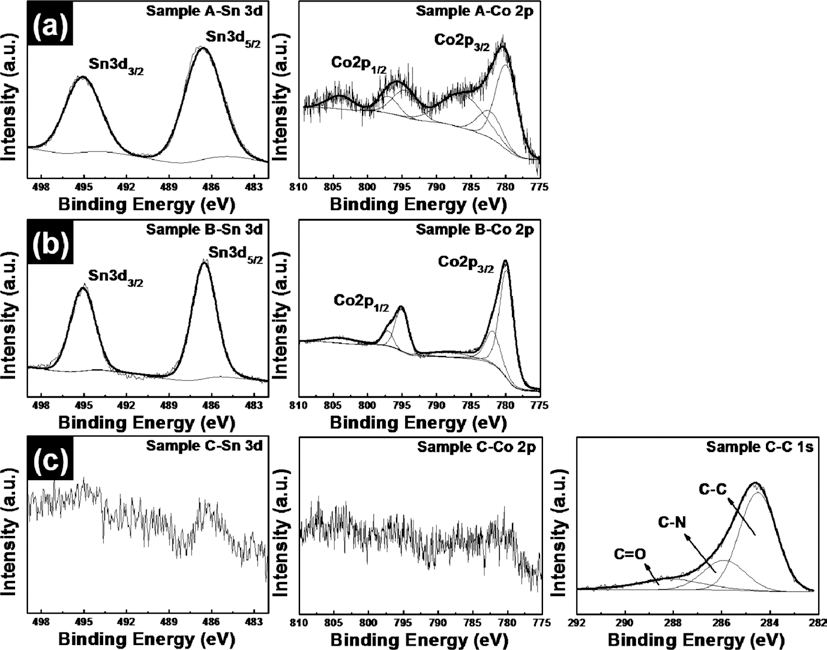
- Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(5):360-365. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.360
- 725 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF SnO2-CoO/carbon-coated CoO core/shell nanowire composites were synthesized by using electrospinning and hydrothermal methods. In order to obtain SnO2-CoO/carbon-coated CoO core/shell nanowire composites, SnO2-Co3O4 nanowire composites and SnO2-Co3O4/polygonal Co3O4 core/shell nanowire composites are also synthesized. To demonstrate their structural, chemical bonding, and morphological properties, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. These results indicated that the morphologies and structures of the samples were changed from SnO2-Co3O4 nanowires having cylindrical structures to SnO2-Co3O4/Co3O4 core/shell nanowires having polygonal structures after a hydrothermal process. At last, SnO2-CoO/carbon-coated CoO core/shell nanowire composites having irregular and high surface area are formed after carbon coating using a polypyrrole (PPy). Also, there occur phases transformation of cobalt phases from Co3O4 to CoO during carbon coating using a PPy under a argon atmosphere.
-
Citations
Citations to this article as recorded by- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
혜란 안, 혜린 강, 효정 선, 지호 한, 효진 안
Korean Journal of Materials Research.2015; 25(12): 672~677. CrossRef - Synthesis of Perforated Polygonal Cobalt Oxides usinga Carbon Nanofiber Template
Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2015; 22(5): 350. CrossRef
- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
- Fabrication of Flake-like LiCoO2 Nanopowders using Electrospinning
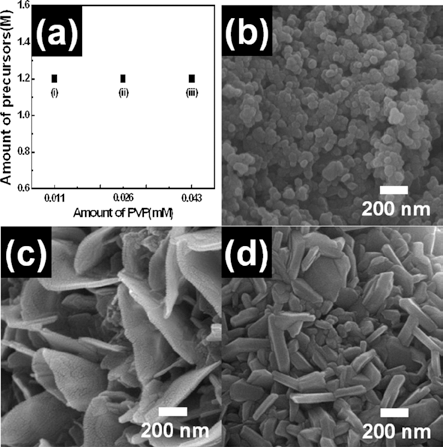
- Bon-Ryul Koo, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(2):108-113. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.108
- 810 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Flake-like LiCoO2 nanopowders were fabricated using electrospinning. To investigate their formation mechanism, field-emssion scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. Among various parameters of electrospinning, we controlled the molar concentration of the precursor and the PVP polymer. When the molar concentration of lithium and cobalt was 0.45 M, the morphology of LiCoO2 nanopowders was irregular and round. For 1.27 M molar concentration, the LiCoO2 nanopowders formed with flake-like morphology. For the PVP polymer, the molar concentration was set to 0.011 mM, 0.026 mM, and 0.043 mM. Irregular LiCoO2 nanopowders were formed at low concentration (0.011 mM), while flake-like LiCoO2 were formed at high concentration (0.026 mM and 0.043 mM). Thus, optimized molar concentration of the precursor and the PVP polymer may be related to the successful formation of flake-like LiCoO2 nanopowders. As a results, the synthesized LiCoO2 nanopowder can be used as the electrode material of Li-ion batteries.
-
Citations
Citations to this article as recorded by- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef - Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 360. CrossRef
- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
TOP
 KPMI
KPMI


 First
First Prev
Prev


