Search
- Page Path
- HOME > Search
- [Korean]
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(4):324-328. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00199

- 1,170 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - This study analyzed the influence of ball size and process control agents on the refinement and dehydrogenation behavior of TiH2 powder. Powders milled using ZrO2 balls with diameters of 0.1 mm, 0.3 mm, and 0.3+0.5+1 mm exhibited a bimodal particle size distribution, of which the first mode had the smallest size of 0.23 μm for the 0.3 mm balls. Using ethanol and/or stearic acid as process control agents was effective in particle refinement. Thermogravimetric analysis showed that dehydrogenation of the milled powder started at a relatively low temperature compared to the raw powder, which is interpreted to have resulted from a decrease in particle size and an increase in defects. The dehydrogenation kinetics of the TiH2 powder were evaluated by the magnitude of peak shift with heating rates using thermogravimetric analysis. The activation energy of the dehydrogenation reaction, calculated from the slope of the Kissinger plot, was measured to be 228.6 kJ/mol for the raw powder and 194.5 kJ/mol for the milled powder. TEM analysis revealed that both the milled and dehydrogenated powders showed an angular shape with a size of about 200 nm.
- [English]
- Eco-Friendly Powder and Particles-Based Triboelectric Energy Harvesters
- Rayyan Ali Shaukat, Jihun Choi, Chang Kyu Jeong
- J Powder Mater. 2023;30(6):528-535. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.528
- 2,051 View
- 38 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
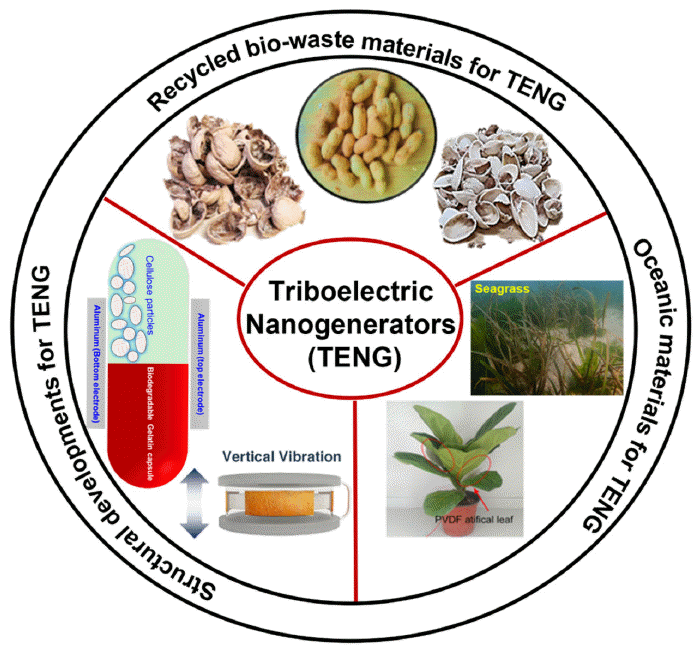
PDF Since their initial development in 2012, triboelectric nanogenerators (TENGs) have gained popularity worldwide as a desired option for harnessing energy. The urgent demand for TENGs is attributed to their novel structural design, low cost, and use of large-scale materials. The output performance of a TENG depends on the surface charge density of the friction layers. Several recycled and biowaste materials have been explored as friction layers to enhance the output performance of TENGs. Natural and oceanic biomaterials have also been investigated as alternatives for improving the performance of TENG devices. Moreover, structural innovations have been made in TENGs to develop highly efficient devices. This review summarizes the recent developments in recycling and biowaste materials for TENG devices. The potential of natural and oceanic biowaste materials is also discussed. Finally, future outlooks for the structural developments in TENG devices are presented.
-
Citations
Citations to this article as recorded by- Triboelectric Nanogenerators for Future Space Missions
Rayyan Ali Shaukat, Muhammad Muqeet Rehman, Maryam Khan, Rui Chang, Carlo Saverio Iorio, Yarjan Abdul Samad, Yijun Shi
Nano-Micro Letters.2026;[Epub] CrossRef - Fabrication and Characterization of a Flexible Polyurethane-Based Triboelectric Nanogenerator for a Harvesting Energy System
Saba Ejaz, Imran Shah, Shahid Aziz, Gul Hassan, Ahmed Shuja, Muhammad Asif Khan, Dong-Won Jung
Micromachines.2025; 16(2): 230. CrossRef - Optimized Process and Mechanical and Electrical Analysis of Polyimide/Pb(Zr,Ti)O3-Based Flexible Piezoelectric Composites
Junki Lee, Sang-il Yoon, Hyunseung Kim, Chang Kyu Jeong
Journal of Powder Materials.2025; 32(1): 16. CrossRef - Sustainable Triboelectric Nanogenerator from Abalone Shell Powder for Self-Powered Humidity Sensing
Yunsook Yang, Farhan Akhtar, Shahzad Iqbal, Muhammad Muqeet Rehman, Woo Young Kim
Sensors.2025; 25(24): 7584. CrossRef - Research Trends in Magneto-Mechano-Electric (MME) Energy Harvesting Devices
So Ie Jeong, Geon-Tae Hwang
Journal of Powder Materials.2025; 32(6): 529. CrossRef
- Triboelectric Nanogenerators for Future Space Missions
- [Korean]
- Partially Dry-Transferred Graphene Electrode with Zinc Oxide Nanopowder and Its Application on Organic Solar Cells
- Yeongsu Jo, Chae Young Woo, Soon Kyu Hong, Hyung Woo Lee
- J Korean Powder Metall Inst. 2020;27(4):305-310. Published online August 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.4.305
- 557 View
- 1 Download
-
 Abstract
Abstract
 PDF
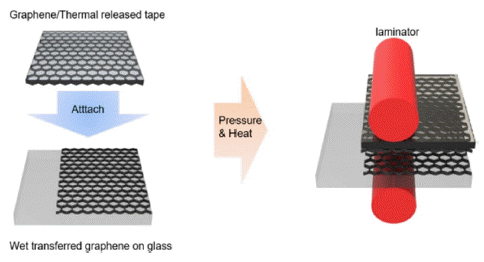
PDF In this study, partially dry transfer is investigated to solve the problem of fully dry transfer. Partially dry transfer is a method in which multiple layers of graphene are dry-transferred over a wet-transferred graphene layer. At a wavelength of 550 nm, the transmittance of the partially dry-transferred graphene is seen to be about 3% higher for each layer than that of the fully dry-transferred graphene. Furthermore, the sheet resistance of the partially drytransferred graphene is relatively lower than that of the fully dry-transferred graphene, with the minimum sheet resistance being 179 Ω/sq. In addition, the fully dry-transferred graphene is easily damaged during the solution process, so that the performance of the organic photovoltaics (OPV) does not occur. In contrast, the best efficiency achievable for OPV using the partially dry-transferred graphene is 2.37% for 4 layers.
- [Korean]
- Recent Development in Performance Enhancement of PVDF-Nanopowder Composite-based Energy Harvesting Devices
- Geon-Ju Choi, Il-Kyu Park
- J Korean Powder Metall Inst. 2020;27(3):247-255. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.247
- 1,150 View
- 11 Download
-
 Abstract
Abstract
 PDF
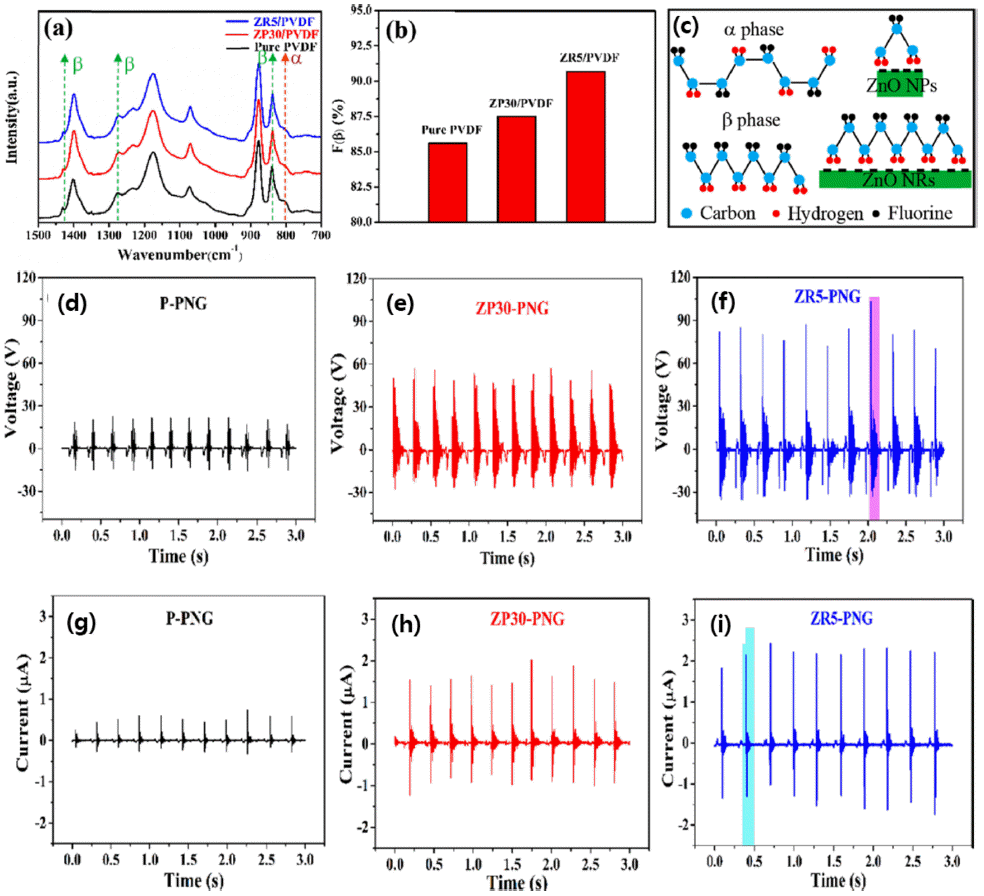
PDF Recently, interest in technology for eco-friendly energy harvesting has been increasing. Polyvinylidene fluoride (PVDF) is one of the most fascinating materials that has been used in energy harvesting technology as well as micro-filters by utilizing an electrostatic effect. To enhance the performance of the electrostatic effect-based nanogenerator, most studies have focused on enlarging the contact surface area of the pair of materials with different triboelectric series. For this reason, one-dimensional nanofibers have been widely used recently. In order to realize practical energy-harvesting applications, PVDF nanofibers are modified by enlarging their contact surface area, modulating the microstructure of the surface, and maximizing the fraction of the β-phase by incorporating additives or forming composites with inorganic nanoparticles. Among them, nanocomposite structures incorporating various nanoparticles have been widely investigated to increase the β-phase through strong hydrogen bonding or ion-dipole interactions with -CF2/CH2- of PVDF as well as to enhance the mechanical strength. In this study, we report the recent advances in the nanocomposite structure of PVDF nanofibers and inorganic nanopowders.
- [Korean]
- Characterization of Classification of Synthesized Ni Nanopowders by Pulsed Wire Evaporation Method
- Joong-Hark Park, Geon-Hong Kim, Dong-Jin Lee, Soon-Jik Hong
- J Korean Powder Metall Inst. 2017;24(5):389-394. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.389
- 641 View
- 4 Download
-
 Abstract
Abstract
 PDF
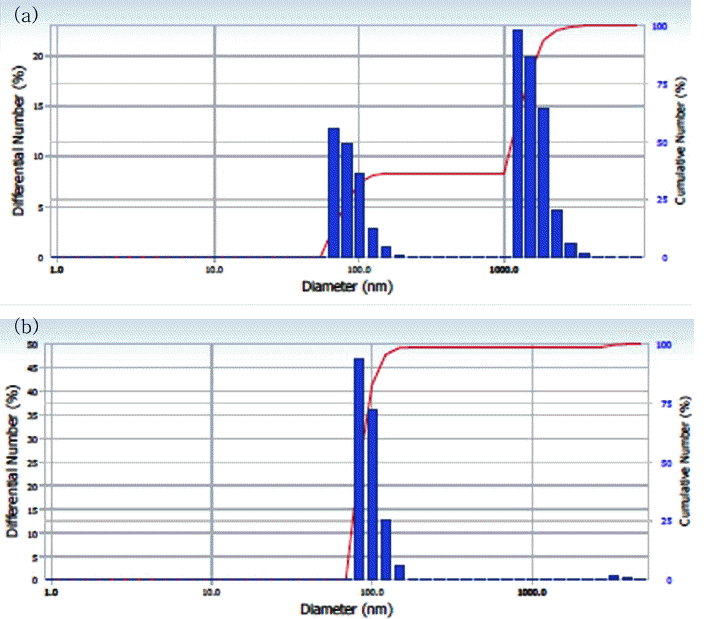
PDF Ni wires with a diameter and length of 0.4 and 100 mm, respectively, and a purity of 99.9% are electrically exploded at 25 cycles per minute. The Ni nanopowders are successfully synthesized by a pulsed wire evaporation (PWE) method, in which Ar gas is used as the ambient gas. The characterization of the nanopowders is carried out using X-ray diffraction (XRD) and a high-resolution transmission electronmicroscope (HRTEM). The Ni nanopowders are classified for a multilayer ceramic condenser (MLCC) application using a type two Air-Centrifugal classifier (model: CNI, MP-250). The characterization of the classified Ni nanopowders are carried out using a scanning electron microscope (SEM) and particle size analysis (PSA) to observe the distribution and minimum classification point (minimum cutting point) of the nanopowders.
- [English]
- Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
- A-Young Lee, Gwang-Yeob Lee, Hye-Ryeong Oh, Hyeon-Ah Kim, Song-Yi Kim, Min-Ha Lee
- J Korean Powder Metall Inst. 2016;23(6):409-413. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.409
- 945 View
- 1 Download
-
 Abstract
Abstract
 PDF
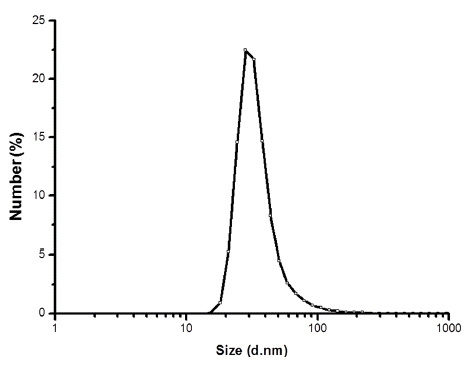
PDF Electrical wire explosion in liquid media is a promising method for producing metallic nanopowders. It is possible to obtain high-purity metallic nanoparticles and uniform-sized nanopowder with excellent dispersion stability using this electrical wire explosion method. In this study, Ni-Fe alloy nanopowders with core-shell structures are fabricated via the electrical explosion of Ni-Fe alloy wires 0.1 mm in diameter and 20 mm in length in de-ionized water. The size and shape of the powders are investigated by field-emission scanning electron microscopy, transmission electron microscopy, and laser particle size analysis. Phase analysis and grain size determination are conducted by X-ray diffraction. The result indicate that a core-shell structured Ni-Fe nanopowder is synthesized with an average particle size of approximately 28 nm, and nanosized Ni core particles are encapsulated by an Fe nanolayer.
- [Korean]
- The Fabrication of Cobalt Nanopowder by Sonochemical Polyol Synthesis of Cobalt Hydroxide and Magnetic Separation Method
- Jong Min Byun, Myoung Hwan Choi, Chang Min Shim, Ji Young Kim, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(1):39-45. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.39

- 815 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this study, cobalt nanopowder is fabricated by sonochemical polyol synthesis and magnetic separation method. First, sonochemical polyol synthesis is carried out at 220°C for up to 120 minutes in diethylene glycol (C4H10O3). As a result, when sonochemical polyol synthesis is performed for 50 minutes, most of the cobalt precursor (Co(OH)2) is reduced to spherical cobalt nanopowder of approximately 100 nm. In particular, aggregation and growth of cobalt particles are effectively suppressed as compared to common polyol synthesis. Furthermore, in order to obtain finer cobalt nanopowder, magnetic separation method using magnetic property of cobalt is introduced at an early reduction stage of sonochemical polyol synthesis when cobalt and cobalt precursor coexist. Finally, spherical cobalt nanopowder having an average particle size of 22 nm is successfully separated.
- [Korean]
- Fabrication of Flake-like LiCoO2 Nanopowders using Electrospinning
- Bon-Ryul Koo, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(2):108-113. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.108
- 816 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
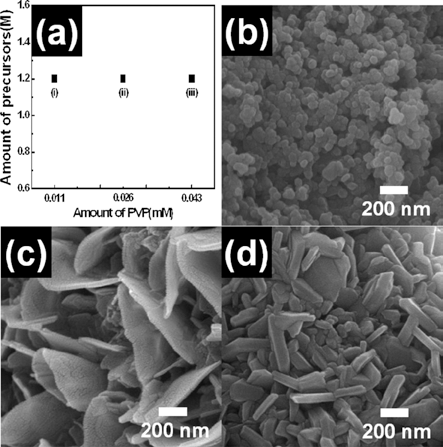
PDF Flake-like LiCoO2 nanopowders were fabricated using electrospinning. To investigate their formation mechanism, field-emssion scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. Among various parameters of electrospinning, we controlled the molar concentration of the precursor and the PVP polymer. When the molar concentration of lithium and cobalt was 0.45 M, the morphology of LiCoO2 nanopowders was irregular and round. For 1.27 M molar concentration, the LiCoO2 nanopowders formed with flake-like morphology. For the PVP polymer, the molar concentration was set to 0.011 mM, 0.026 mM, and 0.043 mM. Irregular LiCoO2 nanopowders were formed at low concentration (0.011 mM), while flake-like LiCoO2 were formed at high concentration (0.026 mM and 0.043 mM). Thus, optimized molar concentration of the precursor and the PVP polymer may be related to the successful formation of flake-like LiCoO2 nanopowders. As a results, the synthesized LiCoO2 nanopowder can be used as the electrode material of Li-ion batteries.
-
Citations
Citations to this article as recorded by- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef - Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 360. CrossRef
- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
TOP
 KPMI
KPMI


 First
First Prev
Prev


