Search
- Page Path
- HOME > Search
- [English]
- Bandgap Tuning and Quenching Effects of In(Zn)P@ZnSe@ZnS Quantum Dots
- Sang Yeon Lee, Su Hyun Park, Gyungsu Byun, Chang-Yeoul Kim
- J Powder Mater. 2024;31(3):226-235. Published online June 27, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00003

- 2,922 View
- 44 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - InP quantum dot (QDs) have attracted researchers’ interest due to their applicability in quantum dot light-emitting displays (QLED) or biomarkers for detecting cancers or viruses. The surface or interface control of InP QD core/shell has substantially increased quantum efficiency, with a quantum yield of 100% reached by introducing HF to inhibit oxide generation. In this study, we focused on the control of bandgap energy of quantum dots by changing the Zn/(In+Zn) ratio in the In(Zn)P core. Zinc incorporation can change the photoluminescent light colors of green, yellow, orange, and red. Diluting a solution of as-synthesized QDs by more than 100 times did not show any quenching effects by the Förster resonance energy transfer phenomenon between neighboring QDs.
-
Citations
Citations to this article as recorded by- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
Hongcheng Yang, Junjie Hao, Mingyu Sun, Yujie Song, Kai Wang, Yujie Song, Xiao Wei Sun, Wenda Zhang
The Innovation.2026; 7(2): 101121. CrossRef
- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
- [Korean]
- Recent Developments in Quantum Dot Patterning Technology for Quantum Dot Display
- Yeong Jun Jin, Kyung Jun Jung, Jaehan Jung
- J Powder Mater. 2024;31(2):169-179. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00073
- 6,930 View
- 153 Download
-
 Abstract
Abstract
 PDF
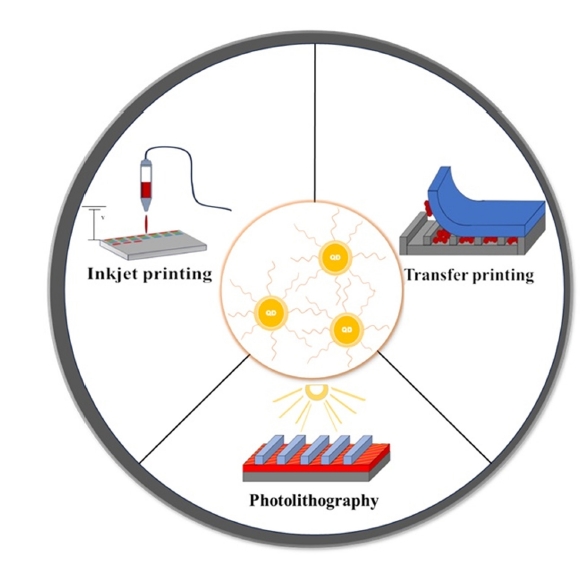
PDF - Colloidal quantum dot (QDs) have emerged as a crucial building block for LEDs due to their size-tunable emission wavelength, narrow spectral line width, and high quantum efficiency. Tremendous efforts have been dedicated to improving the performance of quantum dot light-emitting diodes (QLEDs) in the past decade, primarily focusing on optimization of device architectures and synthetic procedures for high quality QDs. However, despite these efforts, the commercialization of QLEDs has yet to be realized due to the absence of suitable large-scale patterning technologies for high-resolution devices., This review will focus on the development trends associated with transfer printing, photolithography, and inkjet printing, and aims to provide a brief overview of the fabricated QLED devices. The advancement of various quantum dot patterning methods will lead to the development of not only QLED devices but also solar cells, quantum communication, and quantum computers.
- [English]
- Nitric Oxide Detection of Fe(DTC)3-hybrizided CdSe Quantum Dots Via Fluorescence Energy Transfer
- Chang-Yeoul Kim
- J Powder Mater. 2022;29(6):453-458. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.453
- 1,115 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF We successfully synthesize water-dispersible CTAB-capped CdSe@ZnS quantum dots with the crystal size of the CdSe quantum dots controlled from green to orange colors. The quenching effect of Fe(DTC)3 is very efficient to turn off the emission light of quantum dots at four molar ratios of the CdSe quantum dots, that is, the effective covering the surface of quantum dots with Fe(DTC)3. However, the reaction with Fe(DTC)3 for more than 24 h is required to completely realize the quenching effect. The highly quenched quantum dots efficiently detect nitric oxide at nano-molar concentration of 110nM of NO with 34% of recovery of emission light intensity. We suggest that Fe(DTC)3-hybridized CdSe@ZnS quantum dots are an excellent fluorescence resonance energy transfer probe for the detection of nitric oxide in biological systems.
- [Korean]
- Fluorescent Nanoparticles: Synthesis and Applications
- Y. K. Kim, B. K. Song, J. G. Lee, Y. K. Baek
- J Korean Powder Metall Inst. 2020;27(2):154-163. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.154
- 2,844 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
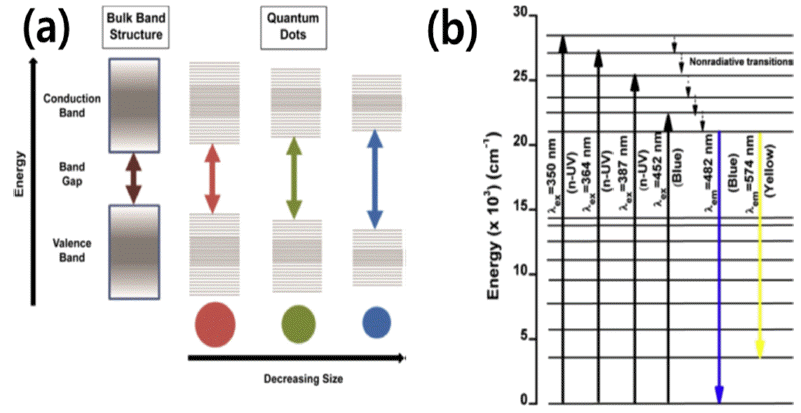
PDF Fluorescent nanoparticles are characterized by their unique properties such as luminescence, optical transparency, and sensitivity to various chemical environments. For example, semiconductor nanocrystals (quantum dots), which are nanophosphors doped with transition metal or rare earth ions, can be classified as fluorescent nanoparticles. Tuning their optical and physico-chemical properties can be carried out by considering and taking advantage of nanoscale effects. For instance, quantum confinement causes a much higher fluorescence with nanoparticles than with their bulk counterparts. Recently, various types of fluorescent nanoparticles have been synthesized to extend their applications to other fields. In this study, State-of-the-art fluorescent nanoparticles are reviewed with emphasis on their analytical and anti-counterfeiting applications and synthesis processes. Moreover, the fundamental principles behind the exceptional properties of fluorescent nanoparticles are discussed.
-
Citations
Citations to this article as recorded by- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
Ji-Won Heo, A-Young Sung
Korean Journal of Materials Research.2022; 32(4): 193. CrossRef
- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
- [Korean]
- Flexible Cu-In-Se Quantum Dot-Sensitized Solar Cells Based on Nanotube Electrodes
- Jae-Yup Kim
- J Korean Powder Metall Inst. 2019;26(1):45-48. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.45
- 533 View
- 2 Download
-
 Abstract
Abstract
 PDF
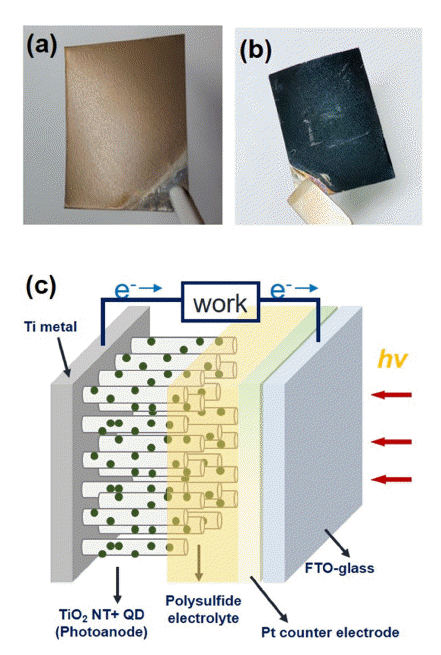
PDF Quantum dots (QDs) are an attractive material for application in solar energy conversion devices because of their unique properties including facile band-gap tuning, a high-absorption coefficient, low-cost processing, and the potential multiple exciton generation effect. Recently, highly efficient quantum dot-sensitized solar cells (QDSCs) have been developed based on CdSe, PbS, CdS, and Cu-In-Se QDs. However, for the commercialization and wide application of these QDSCs, replacing the conventional rigid glass substrates with flexible substrates is required. Here, we demonstrate flexible CISe QDSCs based on vertically aligned TiO2 nanotube (NT) electrodes. The highly uniform TiO2 NT electrodes are prepared by two-step anodic oxidation. Using these flexible photoanodes and semi-transparent Pt counter electrodes, we fabricate the QDSCs and examine their photovoltaic properties. In particular, photovoltaic performances are optimized by controlling the nanostructure of TiO2 NT electrodes.
- [Korean]
- Recent Developments in Synthesis of Colloidal Quantum Dots
- Jae-Yong Jung, Jong-Pal Hong, Young-Kuk Kim
- J Korean Powder Metall Inst. 2018;25(4):346-354. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.346
- 1,857 View
- 12 Download
-
 Abstract
Abstract
 PDF
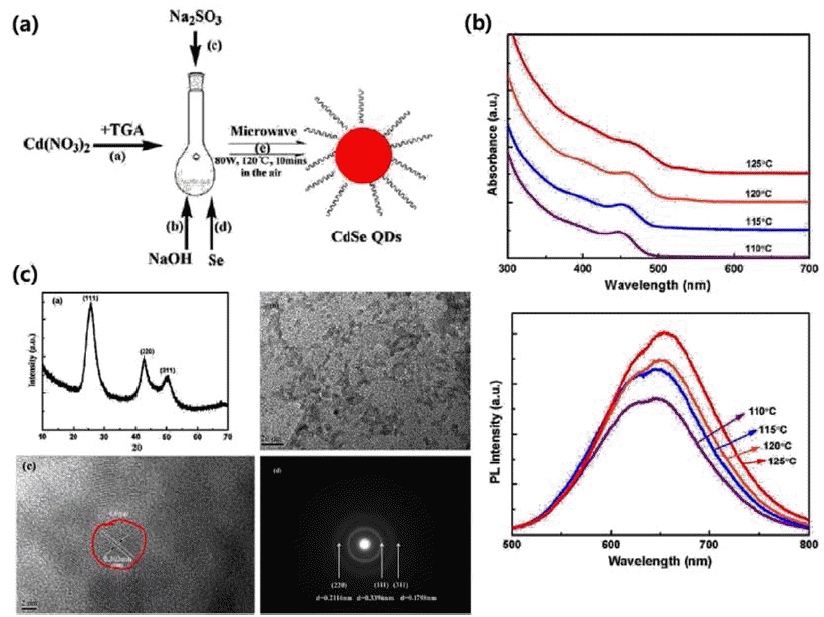
PDF Over the last decade, the study of the synthesis of semiconductor colloidal quantum dots has progressed at a tremendous rate. Colloidal quantum dots, which possess unique spectral-luminescent characteristics, are of great interest in the development of novel materials and devices, which are promising for use in various fields. Several studies have been carried out on hot injection synthesis methods. However, these methods have been found to be unsuitable for large-capacity synthesis. Therefore, this review paper introduces synthesis methods other than the hot injection synthesis method, to synthesize quantum dots with excellent optical properties, through continuous synthesis and large capacity synthesis. In addition, examples of the application of synthesized colloid quantum dots in displays, solar cells, and bio industries are provided.
- [Korean]
- Technology Trend of Luminescent Nanomaterials
- Hyewon Jeong, Jae Sung Son
- J Korean Powder Metall Inst. 2018;25(2):170-177. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.170
- 1,059 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
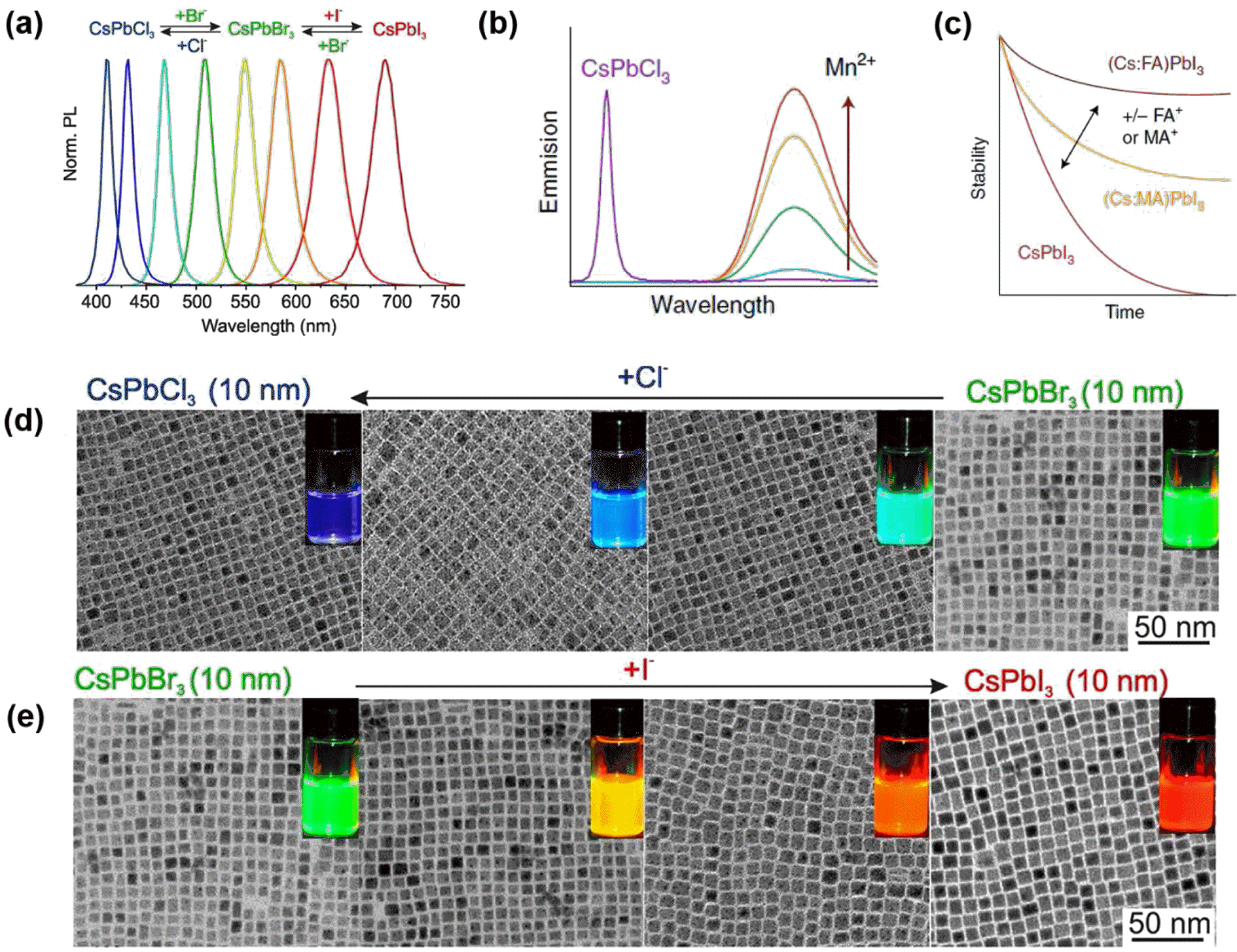
PDF Colloidally synthesized luminescent nanocrystals (NCs) have attracted tremendous attention due to their unique nanoscale optical and electronic properties. The emission properties of these NCs can be precisely tuned by controlling their size, shape, and composition as well as by introducing appropriate dopant impurities. Nowadays, these NCs are actively utilized for various applications such as optoelectronic devices including light emitting diodes (LEDs), lasers, and solar cells, and bio-medical applications such as imaging agents and bio-sensors. In this review, we classify luminescent nanomaterials into quantum dots (QDs), upconversion nanoparticles (UCNPs), and perovskite NCs and present their intrinsic emission mechanism. Furthermore, the recently emerging issues of efficiency, toxicity, and durability in these materials are discussed for better understanding of industry demands. As well, the future outlook will be offered for researchers to guide the direction of future research.
-
Citations
Citations to this article as recorded by- A Structural Relationship between University Dance Students’ Emotional Regulation, Emotion Response, and Engagement in Classes
Jinhee Gong
The Journal of Korean Institute of Information Technology.2020; 18(4): 121. CrossRef
- A Structural Relationship between University Dance Students’ Emotional Regulation, Emotion Response, and Engagement in Classes
- [Korean]
- Synthesis and analysis CdSe/ZnS quantum dot with a Core/shell Continuous Synthesis System Using a Microfluidic Reactor
- Myung Hwan Hong, So Young Joo, Lee-Seung Kang, Chan Gi Lee
- J Korean Powder Metall Inst. 2018;25(2):132-136. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.132
- 1,892 View
- 10 Download
-
 Abstract
Abstract
 PDF
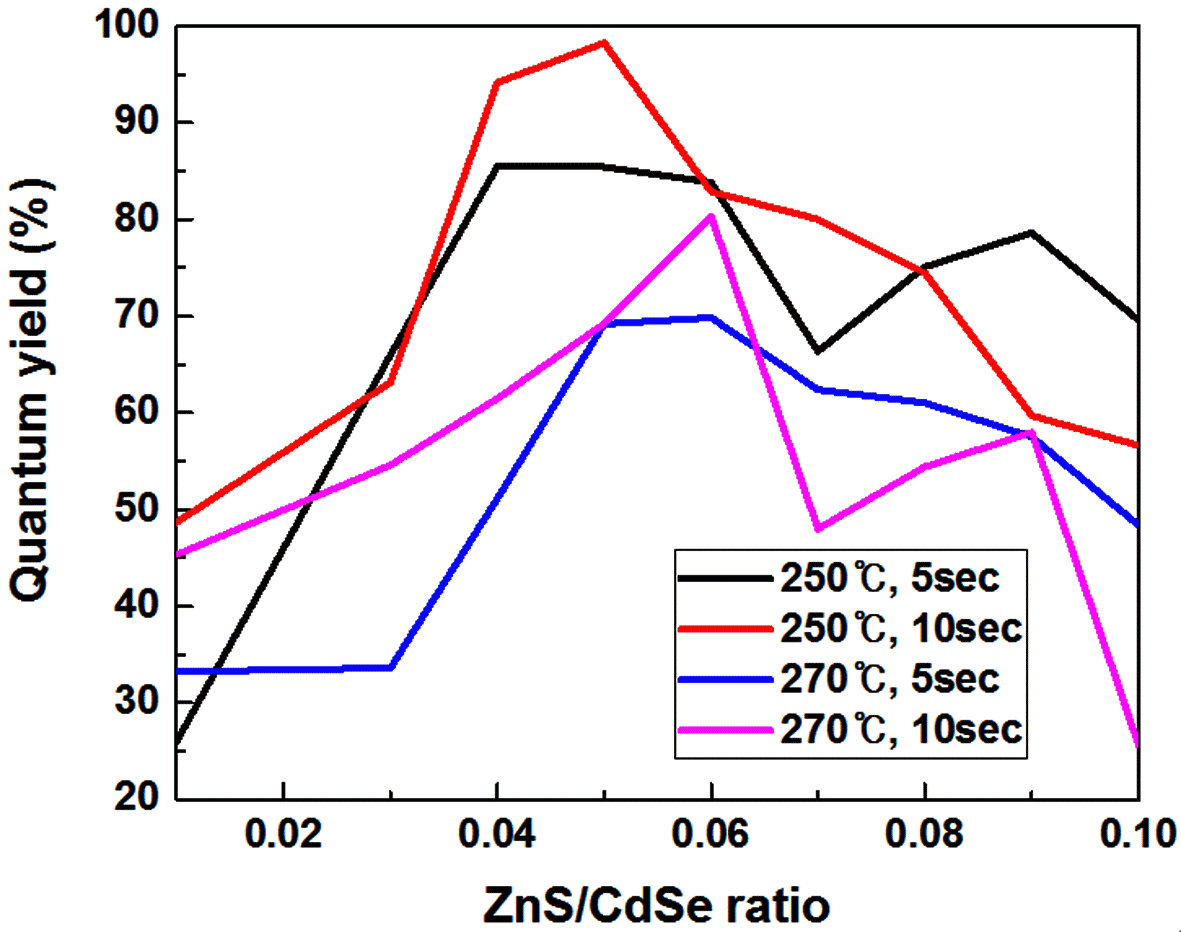
PDF Core/shell CdSe/ZnS quantum dots (QDs) are synthesized by a microfluidic reactor-assisted continuous reactor system. Photoluminescence and absorbance of synthesized CdSe/ZnS core/shell QDs are investigated by fluorescence spectrophotometry and online UV-Vis spectrometry. Three reaction conditions, namely; the shell coating reaction temperature, the shell coating reaction time, and the ZnS/CdSe precursor volume ratio, are combined in the synthesis process. The quantum yield of the synthesized CdSe QDs is determined for each condition. CdSe/ZnS QDs with a higher quantum yield are obtained compared to the discontinuous microfluidic reactor synthesis system. The maximum quantum efficiency is 98.3% when the reaction temperature, reaction time, and ZnS/CdSe ratio are 270°C, 10 s, and 0.05, respectively. Obtained results indicate that a continuous synthesis of the Core/shell CdSe/ZnS QDs with a high quantum efficiency could be achieved by isolating the reaction from the external environment.
- [Korean]
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(6):489-493. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.489
- 1,559 View
- 11 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
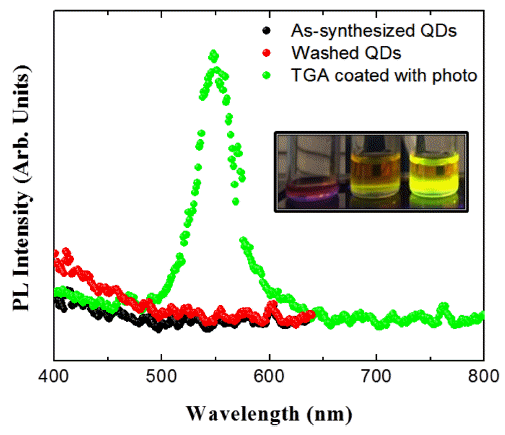
PDF In this study, the surface passivation process for InP-based quantum dots (QDs) is investigated. Surface coating is performed with poly(methylmethacrylate) (PMMA) and thioglycolic acid. The quantum yield (QY) of a PMMA-coated sample slightly increases by approximately 1.3% relative to that of the as-synthesized InP/ZnS QDs. The QYs of the uncoated and PMMA-coated samples drastically decrease after 16 days because of the high defect state density of the InP-based QDs. PMMA does not have a significant effect on the defect passivation. Thioglycolic acid is investigated in this study for the effective surface passivation of InP-based QDs. Surface passivation with thioglycolic acid is more effective than that with the PMMA coating, and the QY increases from 1.7% to 11.3%. ZnS formed on the surface of the InP QDs and S in thioglycolic acid show strong bonding property. Additionally, the QY is further increased up to 21.0% by the photochemical reaction. Electron–hole pairs are formed by light irradiation and lead to strong bonding between the inorganic and thioglycolic acid sulfur. The surface of the InP core QDs, which does not emit light, is passivated by the irradiated light and emits green light after the photochemical reaction.
-
Citations
Citations to this article as recorded by- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef
- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
- [Korean]
- Luminescence Properties of InP/ZnS Quantum Dots depending on InP Core synthesis Temperature
- Han Wook Seo, Da-Woon Jeong, Min Young Kim, Seoung Kyun Hyun, Ji Sun On, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(4):321-325. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.321
- 1,437 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
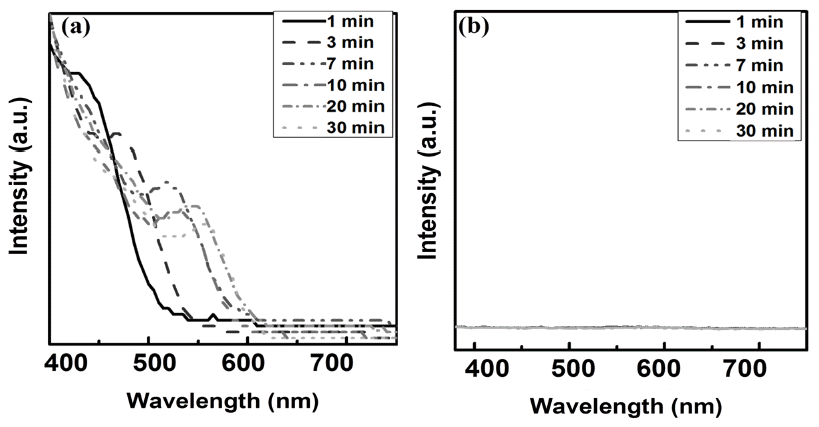
PDF In this study, we investigate the optical properties of InP/ZnS core/shell quantum dots (QDs) by controlling the synthesis temperature of InP. The size of InP determined by the empirical formula tends to increase with temperature: the size of InP synthesized at 140oC and 220oC is 2.46 nm and 4.52 nm, respectively. However, the photoluminescence (PL) spectrum of InP is not observed because of the formation of defects on the InP surface. The growth of InP is observed during the deposition of the shell (ZnS) on the synthesized InP, which is ended up with green-red PL spectrum. We can adjust the PL spectrum and absorption spectrum of InP/ZnS by simply adjusting the core temperature. Thus, we conclude that there exists an optimum shell thickness for the QDs according to the size.
-
Citations
Citations to this article as recorded by- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- [Korean]
- Growth mechanism of InP and InP/ZnS synthesis using colloidal synthesis
- Han wook Seo, Da-woon Jeong, Bin Lee, Seoung kyun Hyun, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(1):6-10. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.6
- 1,425 View
- 5 Download
-
 Abstract
Abstract
 PDF
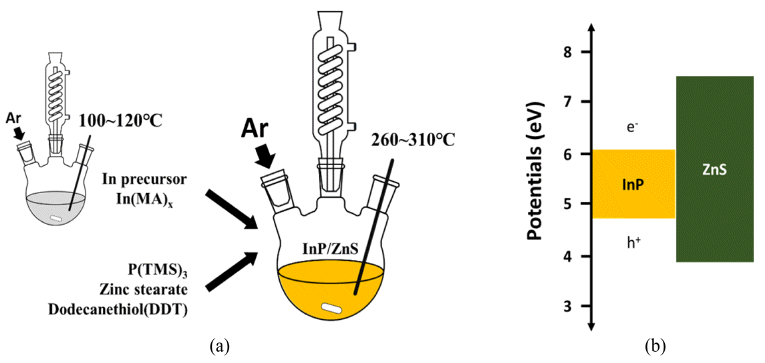
PDF This study investigates the main growth mechanism of InP during InP/ZnS reaction of quantum dots (QDs). The size of the InP core, considering a synthesis time of 1-30 min, increased from the initial 2.56 nm to 3.97 nm. As a result of applying the proposed particle growth model, the migration mechanism, with time index 7, was found to be the main reaction. In addition, after the removal of unreacted In and P precursors from bath, further InP growth (of up to 4.19 nm (5%)), was observed when ZnS was added. The full width at half maximum (FWHM) of the synthesized InP/ZnS quantum dots was found to be relatively uniform, measuring about 59 nm. However, kinetic growth mechanism provides limited information for InP / ZnS core shell QDs, because the surface state of InP changes with reaction time. Further study is necessary, in order to clearly determine the kinetic growth mechanism of InP / ZnS core shell QDs.
- [Korean]
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,000 View
- 8 Download
-
 Abstract
Abstract
 PDF
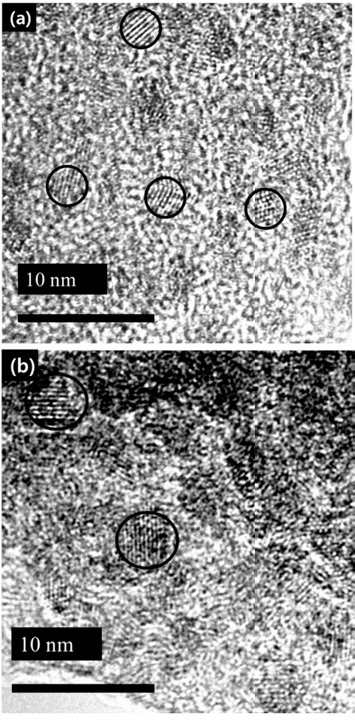
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
- Surface Treatment Method for Long-term Stability of CdSe/ZnS Quantum Dots
- Hyun-Su Park, Da-Woon Jeong, Bum-Sung Kim, So-Yeong Joo, Chan-Gi Lee, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(1):1-5. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.1
- 1,488 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
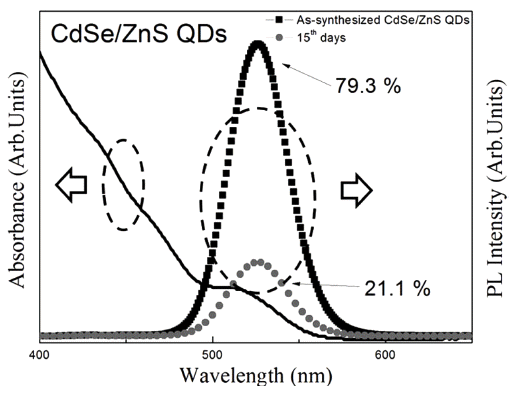
PDF We have investigated the washing method of as-synthesized CdSe/ZnS core/shell structure quantum dots (QDs) and the effective surface passivation method of the washed QDs using PMMA. The quantum yield (QY%) of assynthesized QDs decreases with time, from 79.3% to 21.1%, owing to surface reaction with residual organics. The decreased QY% is restored to the QY% of as-synthesized QDs by washing. However, the QY% of washed QDs also decreases with time, owing to the absence of surface passivation layer. On the other hand, the PMMA-treated QDs maintained a relatively higher QY% after washing than that of the washed QDs that were kept in toluene solution for 30 days. Formation of the PMMA coating layer on CdSe/ZnS QD surface is confirmed by HR-TEM and FT-IR. It is found that the PMMA surface coating, when combined with washing, is useful to be applied in the storage of QDs, owing to its long-term stability.
-
Citations
Citations to this article as recorded by- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
Seung Hwan Ji, Hye Won Yun, Jin Ho Lee, Bum-Sung Kim, Woo-Byoung Kim
Korean Journal of Materials Research.2021; 31(1): 16. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
- [Korean]
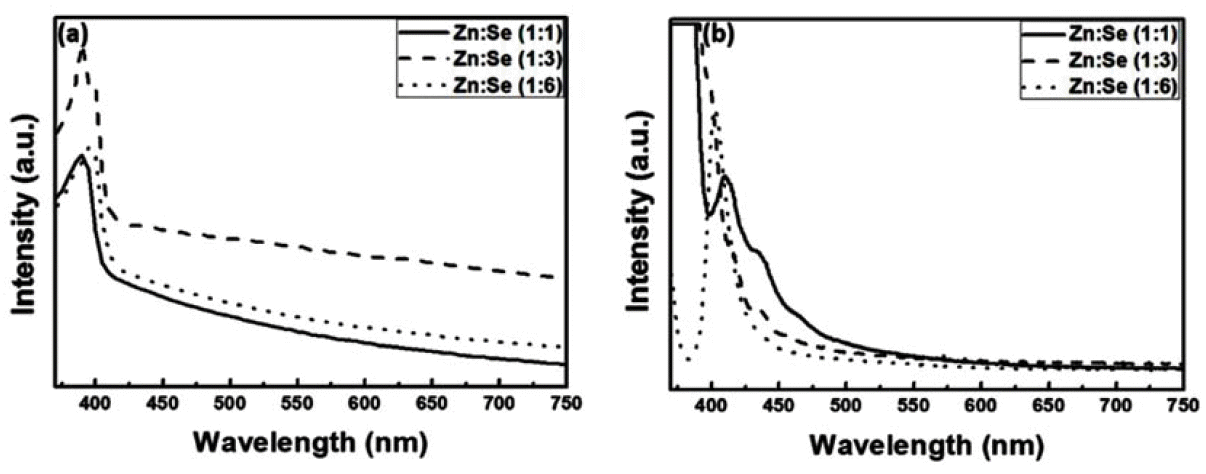
- The Effect of Surface Defects on the Optical Properties of ZnSe:Eu Quantum Dots
- Da-Woon Jeong, Ji Young Park, Han Wook Seo, Kyoung-Mook Lim, Tae-Yeon Seong, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(5):348-352. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.348
- 1,289 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Quantum dots (QDs) are capable of controlling the typical emission and absorption wavelengths because of the bandgap widening effect of nanometer-sized particles. These phosphor particles have been used in optical devices, photovoltaic devices, advanced display devices, and several biomedical complexes. In this study, we synthesize ZnSe QDs with controlled surface defects by a heating-up method. The optical properties of the synthesized particles are analyzed using UV-visible and photoluminescence (PL) measurements. Calculations indicate nearly monodisperse particles with a size of about 5.1 nm at 260°C (full width at half maximum = 27.7 nm). Furthermore, the study results confirm that successful doping is achieved by adding Eu3+ preparing the growth phase of the ZnSe:Eu QDs when heating-up method. Further, we investigate the correlation between the surface defects and the luminescent properties of the QDs.
-
Citations
Citations to this article as recorded by- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
So-Yeong Joo, Hyun-Su Park, Do-yeon Kim, Bum-Sung Kim, Chan Gi Lee, Woo-Byoung Kim
AIP Advances.2018;[Epub] CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
- [Korean]
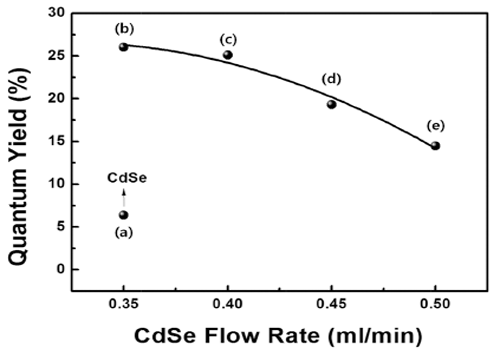
- Optical Characteristics of CdSe/ZnS Quantum Dot with Precursor Flow Rate Synthesized by using Microreactor
- Ji Young Park, Da-Woon Jeong, Won Ju, Han Wook Seo, Yong-Ho Choa, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(2):91-94. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.91
- 1,237 View
- 6 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF High-quality colloidal CdSe/ZnS (core/shell) is synthesized using a continuous microreactor. The particle size of the synthesized quantum dots (QDs) is a function of the precursor flow rate; as the precursor flow rate increases, the size of the QDs decreases and the band gap energy increases. The photoluminescence properties are found to depend strongly on the flow rate of the CdSe precursor owing to the change in the core size. In addition, a gradual shift in the maximum luminescent wave (λmax) to shorter wavelengths (blue shift) is found owing to the decrease in the QD size in accordance with the quantum confinement effect. The ZnS shell decreases the surface defect concentration of CdSe. It also lowers the thermal energy dissipation by increasing the concentration of recombination. Thus, a relatively high emission and quantum yield occur because of an increase in the optical energy emitted at equal concentration. In addition, the maximum quantum yield is derived for process conditions of 0.35 ml/min and is related to the optimum thickness of the shell material.
-
Citations
Citations to this article as recorded by- Quantum materials made in microfluidics - critical review and perspective
M. Wojnicki, V. Hessel
Chemical Engineering Journal.2022; 438: 135616. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- Quantum materials made in microfluidics - critical review and perspective
- [Korean]
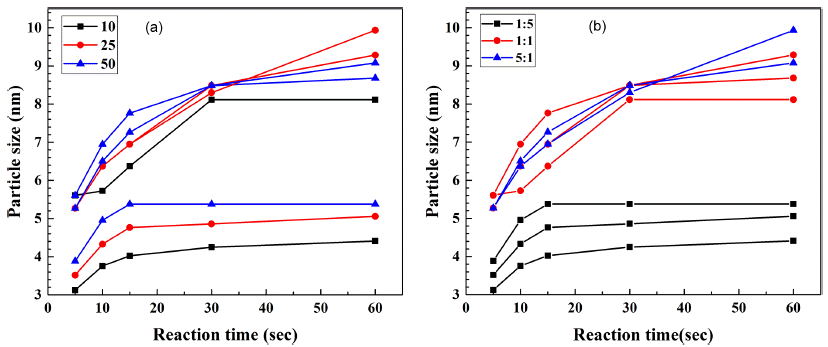
- Synthesis and analysis CdSe Quantum dot with a Microfluidic Reactor Using a Combinatorial Synthesis System
- Myung Hwan Hong, Duk-Hee Lee, Lee-Seung Kang, Chan Gi Lee, Bum-Sung Kim, Nam-Hoon Kim
- J Korean Powder Metall Inst. 2016;23(2):143-148. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.143
- 851 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF A microfluidic reactor with computer-controlled programmable isocratic pumps and online detectors is employed as a combinatorial synthesis system to synthesize and analyze materials for fabricating CdSe quantum dots for various applications. Four reaction condition parameters, namely, the reaction temperature, reaction time, Cd/Se compositional ratio, and precursor concentration, are combined in synthesis condition sets, and the size of the synthesized CdSe quantum dots is determined for each condition. The average time corresponding to each reaction condition for obtaining the ultraviolet–visible absorbance and photoluminescence spectra is approximately 10 min. Using the data from the combinatorial synthesis system, the effects of the reaction conditions on the synthesized CdSe quantum dots are determined. Further, the data is used to determine the relationships between the reaction conditions and the CdSe particle size. This method should aid in determining and selecting the optimal conditions for synthesizing nanoparticles for diverse applications.
- [Korean]
- Improved Luminescent Characterization and Synthesis of InP/ZnS Quantum Dot with High-Stability Precursor
- Eun-Jin Lee, Jong-Woo Moon, Yang-Do Kim, Pyung-Woo Shin, Young-Kuk Kim
- J Korean Powder Metall Inst. 2015;22(6):385-390. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.385
- 1,068 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF We report a synthesis of non-toxic InP nanocrystals using non-pyrolytic precursors instead of pyrolytic and unstable tris(trimethylsilyl)phosphine, a popular precursor for synthesis of InP nanocrystals. In this study, InP nanocrystals are successfully synthesized using hexaethyl phosphorous triamide (HPT) and the synthesized InP nanocrystals showed a broad and weak photoluminescence (PL) spectrum. As synthesized InP nanocrystals are subjected to further surface modification process to enhance their stability and photoluminescence. Surface modification of InP nanocrystals is done at 230°C using 1-dodecanethiol, zinc acetate and fatty acid as sources of ZnS shell. After surface modification, the synthesized InP/ZnS nanocrystals show intense PL spectra centered at the emission wavelength 612 nm through 633 nm. The synthesized InP/ZnS core/shell structure is confirmed with X-ray diffraction (XRD) and Inductively Coupled Plasma - Atomic Emission Spectrometer (ICP-AES). After surface modification, InP/ZnS nanocrystals having narrow particle size distribution are observed by Field Emission Transmission Electron Microscope (FE-TEM). In contrast to uncapped InP nanocrystals, InP/ZnS nanocrystals treated with a newly developed surface modified procedure show highly enhanced PL spectra with quantum yield of 47%.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef - Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(1): 11. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
TOP
 KPMI
KPMI


 First
First Prev
Prev


