Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
- [Korean]
- Formation of Nano-oxides on Porous Metallic Glass Compacts using Hydrothermal Synthesis
- H. J. Park, Y. S. Kim, S. H. Hong, J. T. Kim, J. Y. Cho, W. H. Lee, K. B. Kim
- J Korean Powder Metall Inst. 2015;22(4):229-233. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.229

- 753 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous metallic glass compact (PMGC) are developed by electro-discharge sintering (EDS) process of gas atomized Zr41.2Ti13.8Cu12.5Ni10Be22.5 metallic glass powder under of 0.2 kJ generated by a 450 μF capacitor being charged to 0.94 kV. Functional iron-oxides are formed and growth on the surface of PMGCs via hydrothermal synthesis. It is carried out at 150°C for 48hr with distilled water of 100 mL containing Fe ions of 0.18 g/L. Consequently, two types of iron oxides with different morphology which are disc-shaped Fe2O3 and needle-shaped Fe3O4 are successfully formed on the surface of the PMGCs. This finding suggests that PMGC witih hydrothermal technique can be attractive for the practical technology as a new area of structural and functional materials. And they provide a promising road map for using the metallic glasses as a potential functional application.
-
Citations
Citations to this article as recorded by- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
- [Korean]
- Electromagnetic Wave Shielding Effect of Nano-powder Dispersed Epoxy Resin Composite
- Jun-Young Han, Chul-Hee Lee, Min-Gyu Choi, Soon-Jik Hong, Joong-Hark Park, Dong-Jin Lee
- J Korean Powder Metall Inst. 2015;22(4):234-239. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.234

- 571 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Electronic products are a major part of evolving industry and human life style; however most of them are known to emit electromagnetic waves that have severe health hazards. Therefore, different materials and fabrication techniques are understudy to control or limit transfer of such waves to human body. In this study, nanocomposite powder is dispersed into epoxy resin and shielding effects such as absorption, reflection, penetration and multiple reflections are investigated. In addition, nano size powder (Ni, Fe2O3, Fe-85Ni, C-Ni) is fabricated by pulsed wire evaporation method and dispersed manually into epoxy. Characterization techniques such as X-ray diffraction, Scanning electron microscopy and Transmission electron microscopy are used to investigate the phase analysis, size and shape as well as dispersion trend of a nano powder on epoxy matrix. Shielding effect is measured by standard test method to investigate the electromagnetic shielding effectiveness of planar materials, ASTM D4935. At lower frequency, sample consisting nano-powder of Fe-85%Wt Ni shows better electromagnetic shielding effect compared to only epoxy, only Ni, Fe2O3 and C-Ni samples.
- [Korean]
- Shape Control of Anodic Aluminum Oxide and Effect as Support of Silicon Powder Electrode
- Ju-Seok Song, Jong-Keun Ha, Yoo-Young Kim, Dong-Kyu Park, In-Shup Ahn, Jou-Hyeon Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2015;22(4):240-246. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.240

- 747 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Anodic aluminum oxide (AAO) has been widely used for the development and fabrication of nano-powder with various morphologies such as particle, wire, rod, and tube. So far, many researchers have reported about shape control and fabrication of AAO films. However, they have reported on the shape control with different diameter and length of anodic aluminum oxide mainly. We present a combined mild-hard (or hard-mild) anodization to prepare shape-controlled AAO films. Two main parameters which are combination mild-hard (or hard-mild) anodization and run-time of voltage control are applied in this work. The voltages of mild and hard anodization are respectively 40 and 80 V. Anodization was conducted on the aluminum sheet in 0.3 mole oxalic acid at 4°C. AAO films with morphologies of varying interpore distance, branch-shaped pore, diameter-modulated pore and long funnel-shaped pore were fabricated. Those shapes will be able to apply to fabricate novel nano-materials with potential application which is especially a support to prevent volume expansion of inserted active materials, such as metal silicon or tin powder, in lithium ion battery. The silicon powder electrode using an AAO as a support shows outstanding cycle performance as 1003 mAh/g up to 200 cycles.
-
Citations
Citations to this article as recorded by- Nano silicon encapsulated in modified copper as an anode for high performance lithium ion battery
Jong-Keun Ha, Anupriya K. Haridas, Gyu-Bong Cho, Hyo-Jun Ahn, Jou-Hyeon Ahn, Kwon-Koo Cho
Applied Surface Science.2019; 481: 307. CrossRef
- Nano silicon encapsulated in modified copper as an anode for high performance lithium ion battery
- [English]
- Atomistic Simulation of Sintering Mechanism for Copper Nano-Powders
- Yujin Seong, Sungwon Hwang, See Jo Kim, Sungho Kim, Seong-Gon Kim, Hak Jun Kim, Seong Jin Park
- J Korean Powder Metall Inst. 2015;22(4):247-253. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.247

- 1,567 View
- 12 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The sintering mechanisms of nanoscale copper powders have been investigated. A molecular dynamics (MD) simulation with the embedded-atom method (EAM) was employed for these simulations. The dimensional changes for initial-stage sintering such as characteristic lengths, neck growth, and neck angle were calculated to understand the densification behavior of copper nano-powders. Factors affecting sintering such as the temperature, powder size, and crystalline misalignment between adjacent powders have also been studied. These results could provide information of setting the processing cycles and material designs applicable to nano-powders. In addition, it is expected that MD simulation will be a foundation for the multi-scale modeling in sintering process.
-
Citations
Citations to this article as recorded by- Mesoscale modelling of polymer powder densification due to thermal sintering
Amine Bahloul, Issam Doghri, Laurent Adam
Applied Mathematical Modelling.2023; 114: 408. CrossRef - Review of “Integrated Computer-Aided Process Engineering Session in the International Symposium on Innovation in Materials Processing (ISIMP, 26–29 October 2021)”
Hyunjoo Choi, Jungjoon Kim, Pil-Ryung Cha, Hyoung Seop Kim
MATERIALS TRANSACTIONS.2023; 64(10): 2542. CrossRef - Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
Eun Byeol Choi, Jong-Hyun Lee
Applied Surface Science.2017; 415: 67. CrossRef
- Mesoscale modelling of polymer powder densification due to thermal sintering
- [Korean]
- Thermoelectric Properties in the Cu Doping Effects of the n-type Bi-Te Powders
- Min Soo Park, Hye Young Koo, Gook Hyun Ha, Yong Ho Park
- J Korean Powder Metall Inst. 2015;22(4):254-259. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.254

- 787 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF Bi2Te3 related compounds show the best thermoelectric properties at room temperature. However, n-type Bi2Te2.7Se0.3 showed no improvement on ZT values. To improve the thermolectric propterties of n-type Bi2Te2.7Se0.3, this research has Cu-doped n-type powder. This study focused on effects of Cu-doping method on the thermoelectric properties of n-type materials, and evaluated the comparison between the Cu chemical and mechanical doping. The synthesized powder was manufactured by the spark plasma sintering(SPS). The thermoelectric properties of the sintered body were evaluated by measuring their Seebeck coefficient, electrical resistivity, thermal conductivity, and hall coefficient. An introduction of a small amount of Cu reduced the thermal conductivity and improved the electrical properties with Seebeck coefficient. The authors provided the optimal concentration of Cu0.1Bi1.99Se0.3Te2.7. A figure of merit (ZT) value of 1.22 was obtained for Cu0.1Bi1.9Se0.3Te2.7 at 373K by Cu chemical doping, which was obviously higher than those of Cu0.1Bi1.9Se0.3Te2.7 at 373K by Cu mechanical doping (ZT=0.56) and Cu-free Bi2Se0.3Te2.7 (ZT=0.51).
- [Korean]
- Preparation of Spherical Cobalt Fine Powders by New Liquid Reduction Method
- Dae Weon Kim, Ji-Hoon Kim, Yo-Han Choi, Hee Lack Choi, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2015;22(4):260-265. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.260
- 752 View
- 5 Download
-
 Abstract
Abstract
 PDF
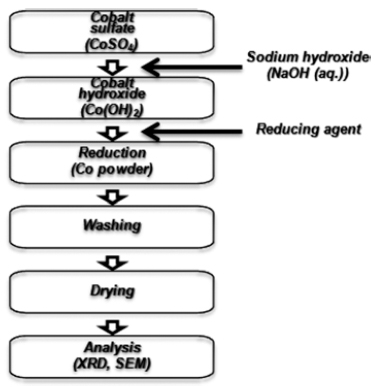
PDF Spherical fine cobalt powders were fabricated by new liquid reduction method. Commercial cobalt sufate heptahydrate was used as raw material. Also ethylene glycol was used as solvent and hydrazine-sodium hypophosphite mixture was used as reduction agent for the new liquid reduction method. A plate shaped cobalt powders with an approximately 300 nm were prepared by a traditional wet ruduction method using distilled water as solvent and hydrazine. Spherical fine cobalt powders with an average size of 1-3 μm were synthesized by a new liquid reduction method in 0.3M cobalt sulfate and 1.5M hydrazine-0.6M sodium hypophosphite mixture at 333K.
- [Korean]
- Synthesis of NiTi Alloy Powder by the Reaction of NiO-TiH2 Mixing Powders
- Ki Cheol Jeon, Han-Eol Lee, Da-Mi Yim, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(4):266-270. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.266
- 811 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
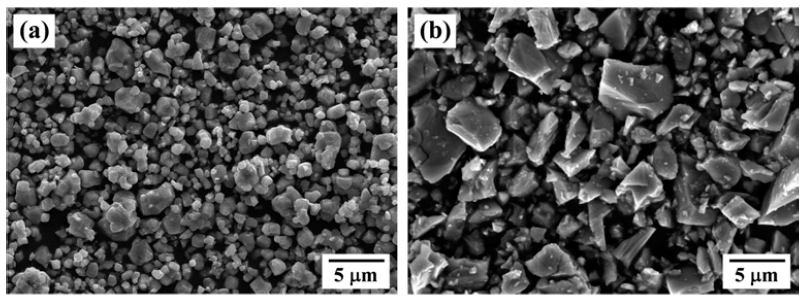
PDF The synthesis of NiTi alloy powders by hydrogen reduction and dehydrogenation process of NiO and TiH2 powder mixtures is investigated. Mixtures of NiO and TiH2 powders are prepared by simple mixing for 1 h or ball milling for 24 h. Simple-mixed mixture shows that fine NiO particles are homogeneously coated on the surface of TiH2 powders, whereas ball milled one exhibits the morphology with mixing of fine NiO and TiH2 particles. Thermogravimetric analysis in hydrogen atmosphere reveals that the NiO and TiH2 phase are changed to metallic Ni and Ti in the temperature range of 260 to 290°C and 553 to 639°C, respectively. In the simple-mixed powders by heat-up to 700°C, agglomerates with solid particles and solidified liquid phase are observed, and the size of agglomerates is increased at 1000°C. From the XRD analysis, the presence of liquid phase is explained by the formation and melting of NiTi2 intermetallic compound due to an exothermic reaction between Ni and Ti. The simple-mixed powders, heated to 1000°C, lead to the formation of NiTi phase but additional Ni-, Ti-rich and Ti-oxide phases. In contrast, the microstructure of ball-milled powders is characterized by the neck-grown particles, forming Ni3Ti, Ti-oxide and unreacted Ni phase.
-
Citations
Citations to this article as recorded by- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
Ki Cheol Jeon, Sung Hyun Park, Na-Yeon Kwon, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 303. CrossRef
- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
- [Korean]
- The Effect of Oxides Additives on Anti-corrosion Properties of Sintered 316L Stainless Steel
- Jong-Pil Lee, Ji-Hyun Hong, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2015;22(4):271-277. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.271

- 597 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF As wrought stainless steel, sintered stainless steel (STS) has excellent high-temperature anti-corrosion even at high temperature of 800ºC and exhibit corrosion resistance in air. The oxidation behavior and oxidation mechanism of the sintered 316L stainless was reported at the high temperature in our previous study. In this study, the effects of additives on high-temperature corrosion resistances were investigated above 800ºC at the various oxides (SiO2, Al2O3, MgO and Y2O3) added STS respectively as an oxidation inhibitor. The morphology of the oxide layers were observed by SEM and the oxides phase and composition were confirmed by XRD and EDX. As a result, the weight of STS 316L sintered body increased sharply at 1000oC and the relative density of specimen decreased as metallic oxide addition increased. Compared with STS 316L sintered parts, weight change ratio corresponding to different oxidation time at 900oC and 1000oC, decreased gradually with the addition of metallic oxide. The best corrosion resistance properties of STS could be improved in case of using Y2O3. The oxidation rate was diminished dramatically by suppression the peeling on oxide layers at Y2O3 added sintered stainless steel.
- [Korean]
- Influence of Oxidation Temperatures on the Structure and the Microstructure of GaN MOCVD Scraps
- Hyun Seon Hong, Joong Woo Ahn
- J Korean Powder Metall Inst. 2015;22(4):278-282. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.278

- 1,015 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The GaN-powder scrap generated in the manufacturing process of LED contains significant amounts of gallium. This waste can be an important resource for gallium through recycling of scraps. In the present study, the influence of annealing temperatures on the structural properties of GaN powder was investigated when the waste was recycled through the mechanochemical oxidation process. The annealing temperature varied from 200°C to 1100°C and the changes in crystal structure and microstructure were studied. The annealed powder was characterized using various analytical tools such as TGA, XRD, SEM, and XRF. The results indicate that GaN structure was fully changed to Ga2O3 structure when annealed above 900°C for 2 h. And, as the annealing temperature increased, crystallinity and particle size were enhanced. The increase in particle size of gallium oxide was possibly promoted by powder-sintering which merged particles to larger than 50 nm.
-
Citations
Citations to this article as recorded by- High-temperature thermo-mechanical behavior of functionally graded materials produced by plasma sprayed coating: Experimental and modeling results
Kang Hyun Choi, Hyun-Su Kim, Chang Hyun Park, Gon-Ho Kim, Kyoung Ho Baik, Sung Ho Lee, Taehyung Kim, Hyoung Seop Kim
Metals and Materials International.2016; 22(5): 817. CrossRef
- High-temperature thermo-mechanical behavior of functionally graded materials produced by plasma sprayed coating: Experimental and modeling results
- [Korean]
- Pressureless Sintering and Spark-Plasma Sintering of Fe-TiC Composite Powders
- B. H. Lee, S. W. Bae, S. W. Bae, H. X. Khoa, J. S. Kim
- J Korean Powder Metall Inst. 2015;22(4):283-288. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.283

- 791 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Two sintering methods of a pressureless sintering and a spark-plasma sintering are tested to densify the Fe- TiC composite powders which are fabricated by high-energy ball-milling. A powder mixture of Fe and TiC is prepared in a planetary ball mill at a rotation speed of 500 rpm for 1h. Pressureless sintering is performed at 1100, 1200 and 1300°C for 1-3 hours in a tube furnace under flowing argon gas atmosphere. Spark-plasma sintering is carried out under the following condition: sintering temperature of 1050°C, soaking time of 10 min, sintering pressure of 50 MPa, heating rate of 50°C, and in a vacuum of 0.1 Pa. The curves of shrinkage and its derivative (shrinkage rate) are obtained from the data stored automatically during sintering process. The densification behaviors are investigated from the observation of fracture surface and cross-section of the sintered compacts. The pressureless-sintered powder compacts show incomplete densification with a relative denstiy of 86.1% after sintering at 1300°C for 3h. Spark-plasma sintering at 1050°C for 10 min exhibits nearly complete densification of 98.6% relative density under the sintering pressure of 50 MPa.
-
Citations
Citations to this article as recorded by- Experimental investigation on thermal behaviour of copper-added P/M iron materials at different sintering temperatures
T. K. Kandavel, S. Dhasarathy
Australian Journal of Mechanical Engineering.2021; 19(1): 57. CrossRef
- Experimental investigation on thermal behaviour of copper-added P/M iron materials at different sintering temperatures
- [Korean]
- Technology Trend Analysis of CO2 Capture and Storage by Patent Information
- Su-Jin Lee, Yun-Seock Lee, Jeong-Gu Lee, Soon-Jik Hong, Joong-Beom Lee
- J Korean Powder Metall Inst. 2015;22(4):289-297. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.289
- 695 View
- 3 Download
-
 Abstract
Abstract
 PDF
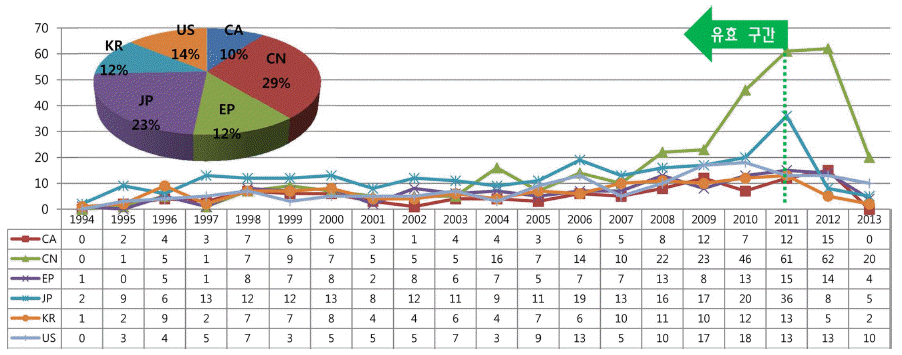
PDF As recognized by all scientific and industrial groups, carbon dioxide(CO2) capture and storage(CCS) could play an important role in reducing greenhouse gas emissions. Especially carbon capture technology by dry sorbent is considered as a most energy-efficient method among the existing CCS technologies. Patent analysis has been considered to be a necessary step for identifying technological trend and planning technology strategies. This paper is aimed at identifying evolving technology trend and key indicators of dry sorbent from the objective information of patents. And technology map of key patents is also presented. In this study the patents applied in korea, japan, china, canada, US, EU from 1993 to 2013 are analyzed. The result of patent analysis could be used for R&D and policy making of domestic CCS industry.
TOP
 KPMI
KPMI




 First
First Prev
Prev


