Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
- [English]
- Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
- A-Young Lee, Gwang-Yeob Lee, Hye-Ryeong Oh, Hyeon-Ah Kim, Song-Yi Kim, Min-Ha Lee
- J Korean Powder Metall Inst. 2016;23(6):409-413. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.409
- 890 View
- 1 Download
-
 Abstract
Abstract
 PDF
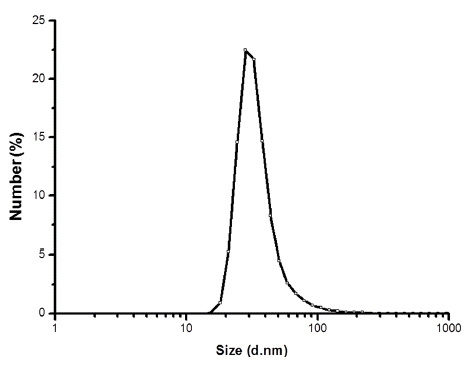
PDF Electrical wire explosion in liquid media is a promising method for producing metallic nanopowders. It is possible to obtain high-purity metallic nanoparticles and uniform-sized nanopowder with excellent dispersion stability using this electrical wire explosion method. In this study, Ni-Fe alloy nanopowders with core-shell structures are fabricated via the electrical explosion of Ni-Fe alloy wires 0.1 mm in diameter and 20 mm in length in de-ionized water. The size and shape of the powders are investigated by field-emission scanning electron microscopy, transmission electron microscopy, and laser particle size analysis. Phase analysis and grain size determination are conducted by X-ray diffraction. The result indicate that a core-shell structured Ni-Fe nanopowder is synthesized with an average particle size of approximately 28 nm, and nanosized Ni core particles are encapsulated by an Fe nanolayer.
- [Korean]
- Synthesis of Hexagonal Boron Nitride Nanocrystals and Their Application to Thermally Conductive Composites
- Jae-Yong Jung, Yang-Do Kim, Pyung-Woo Shin, Young-Kuk Kim
- J Korean Powder Metall Inst. 2016;23(6):414-419. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.414

- 1,465 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF Much attention has been paid to thermally conductive materials for efficient heat dissipation of electronic devices to maintain their functionality and to support lifetime span. Hexagonal boron nitride (h-BN), which has a high thermal conductivity, is one of the most suitable materials for thermally conductive composites. In this study, we synthesize h-BN nanocrystals by pyrolysis of cost-effective precursors, boric acid, and melamine. Through pyrolysis at 900°C and subsequent annealing at 1500°C, h-BN nanoparticles with diameters of ~80 nm are synthesized. We demonstrate that the addition of small amounts of Eu-containing salts during the preparation of melamine borate precursors significantly enhanced the crystallinity of h-BN. In particular, addition of Eu assists the growth of h-BN nanoplatelets with diameters up to ~200 nm. Polymer composites containing both spherical Al2O3 (70 vol%) and Eu-doped h-BN nanoparticles (4 vol%) show an enhanced thermal conductivity (λ ~ 1.72W/mK), which is larger than the thermal conductivity of polymer composites containing spherical Al2O3 (70 vol%) as the sole fillers (λ ~ 1.48W/mK).
- [Korean]
- Synthesis of Nitrogen-doped Carbon Nanofibers for Oxygen Reduction Reaction
- Geon-Hyoung An, Eun-Hwan Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(6):420-425. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.420

- 603 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF N-doped carbon nanofibers as catalysts for oxygen-reduction reactions are synthesized using electrospinning and carbonization. Their morphologies, structures, chemical bonding states, and electrochemical performance are characterized. The optimized N-doped carbon nanofibers exhibit graphitization of carbon nanofibers and an increased nitrogen doping as well as a uniform network structure. In particular, the optimized N-doped carbon nanofibers show outstanding catalytic activity for oxygen-reduction reactions, such as a half-wave potential (E1/2) of 0.43 V, kinetic limiting current density of 6.2 mA cm-2, electron reduction pathways (n = 3.1), and excellent long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 13 mV. The improvement in the electrochemical performance results from the synergistic effect of the graphitization of carbon nanofibers and the increased amount of nitrogen doping.
- [Korean]
- A Study on the Development of Compactability and Electrical Resistivity for P/M Fecralloy
- Jin-Woo Parka, Byung-Hyun Ko, Woo-Young Jung, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2016;23(6):426-431. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.426
- 572 View
- 2 Download
-
 Abstract
Abstract
 PDF
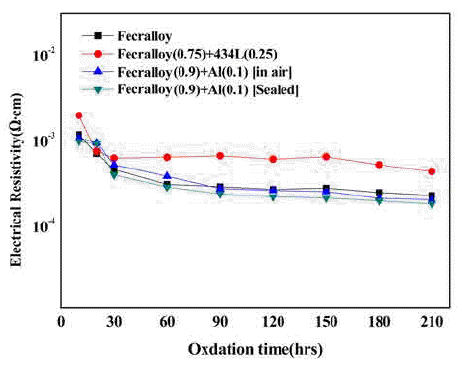
PDF The Fe-Cr-Al alloy system shows an excellent heat resistance because of the formation of an Al2O3 film on the metal surface in an oxidizing atmosphere at high temperatures up to 1400°C. The Fecralloy needs an additive that can act as a binder because of its bad compactability. In this study, the green compacts of STS434L and Al powder added to Fecralloy are oxidized at 950°C for up to 210 h. Fecralloy and Al is mixed by two types of ball milling. One is vented to air and the other was performed in a sealed jar. In the case of Al addition, there are no significant changes in the electrical resistance. Before the oxidation test, Al oxides are present in the Fecralloy surface, as determined from the energy dispersive spectroscopy results. The addition of Al improves the compactability because of an increased density, and the addition of STS434L increases the electrical resistivity by forming a composite oxide.
- [Korean]
- Effect of Cu/Al powder mixing on Dy diffusion in Nd-Fe-B sintered magnets treated with a grain boundary diffusion process
- Min Woo Lee, Tae Suk Jang
- J Korean Powder Metall Inst. 2016;23(6):432-436. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.432
- 745 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
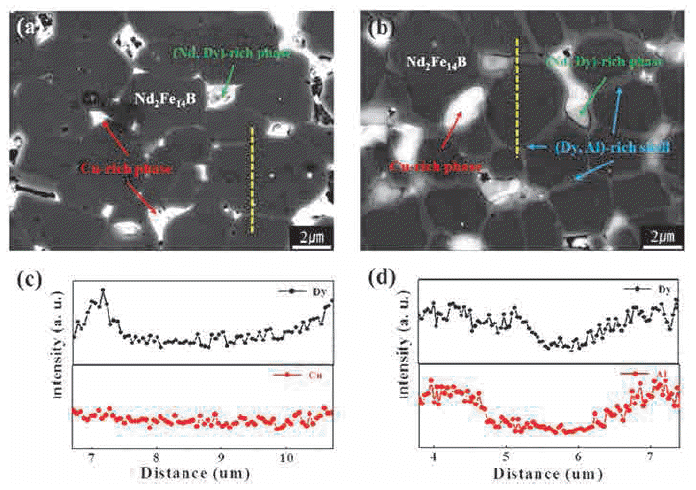
PDF We investigate the microstructural and magnetic property changes of DyH2, Cu + DyH2, and Al + DyH2 diffusion-treated NdFeB sintered magnets with the post annealing (PA) temperature. The coercivity of all the diffusiontreated magnets increases with increasing heat treatment temperature except at 910°C, where it decreases slightly. Moreover, at 880°C, the coercivity increases by 3.8 kOe in Cu and 4.7 kOe in Al-mixed DyH2-coated magnets, whereas this increase is relatively low (3.0 kOe) in the magnet coated with only DyH2. Both Cu and Al have an almost similar effect on the coercivity improvement, particularly over the heat treatment temperature range of 790-880°C. The diffusivity and diffusion depth of Dy increases in those magnets that are treated with Cu or Al-mixed DyH2, mainly because of the comparatively easy diffusion path provided by Cu and Al owing to their solubility in the Nd-rich grain boundary phase. The formation of a highly anisotropic (Nd, Dy)2Fe14B phase layer, which acts as the shell in the core-shell-type structure so as to prevent the reverse domain movement, is the cause of enhanced coercivity of diffusion-treated Nd-Fe-B magnets.
-
Citations
Citations to this article as recorded by- Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
- [Korean]
- Synthesis of DyF3 paste and Magnetic Properties of GBDPed Nd-Fe-B Magnets
- Kwang-Won Jeon, Hee-Ryoung Cha, Jung-Goo Lee
- J Korean Powder Metall Inst. 2016;23(6):437-441. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.437
- 560 View
- 2 Download
-
 Abstract
Abstract
 PDF
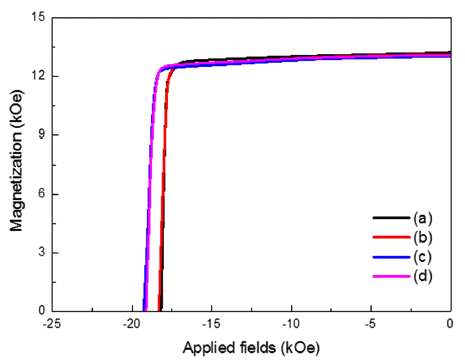
PDF Recently, the grain boundary diffusion process (GBDP), involving heavy rare-earth elements such as Dy and Tb, has been widely used to enhance the coercivity of Nd-Fe-B permanent magnets. For example, a Dy compound is coated onto the surface of Nd-Fe-B sintered magnets, and then the magnets are heat treated. Subsequently, Dy diffuses into the grain boundaries of Nd-Fe-B magnets, forming Dy-Fe-B or Nd-Dy-Fe-B. The dip-coating process is also used widely instead of the GBDP. However, it is quite hard to control the thickness uniformity using dip coating. In this study, first, a DyF3 paste is fabricated using DyF3 powder. Subsequently, the fabricated DyF3 paste is homogeneously coated onto the surface of a Nd-Fe-B sintered magnet. The magnet is then subjected to GBDP to enhance its coercivity. The weight ratio of binder and DyF3 powder is controlled, and we find that the coercivity enhances with decreasing binder content. In addition, the maximum coercivity is obtained with the paste containing 70 wt% of DyF3 powder.
- [Korean]
- Effect of Reaction Parameters on Silica Nanoparticles Synthesized by Sol-gel Method
- Young-Hyun Lim, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2016;23(6):442-446. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.442
- 2,872 View
- 46 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
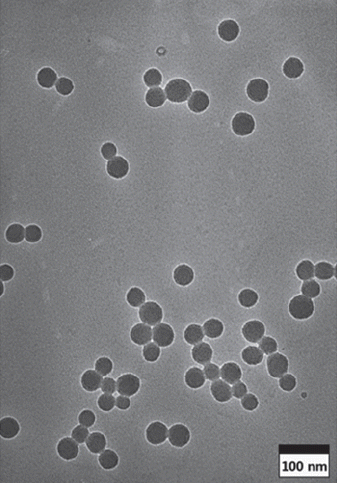
PDF The sol-gel method is the simplest method for synthesizing monodispersed silica particles. The purpose of this study is to synthesize uniform, monodisperse spherical silica nanoparticles using tetraethylorthosilicate (TEOS) as the silica precursor, ethanol, and deionized water in the presence of ammonia as a catalyst. The reaction time and temperature and the concentration of the reactants are controlled to investigate the effect of the reaction parameters on the size of the synthesized particles. The size and morphology of the obtained silica particles are investigated using transmission electron microscopy and particle size analysis. The results show that monodispersed silica particles over a size range of 54-504 nm are successfully synthesized by the sol-gel method without using any additional process. The nanosized silica particles can be synthesized at higher TEOS/H2O ratios, lower ammonia concentrations, and especially, higher reaction temperatures.
-
Citations
Citations to this article as recorded by- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
Mustapha Sulaiman, Naseer Inuwa Durumin Iya, Mamudu Aliyu
FUDMA JOURNAL OF SCIENCES.2024; 8(3): 222. CrossRef - Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 420. CrossRef
- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
- [Korean]
- Microstructure and Magnetic Properties of Nd-Fe-B Sintered Magnet with the Variation of Particle Size
- Dongwon Shin, Dong-Hwan Kim, Young-Cheol Park, Jeong-Gon Kim
- J Korean Powder Metall Inst. 2016;23(6):447-452. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.447
- 611 View
- 5 Download
-
 Abstract
Abstract
 PDF
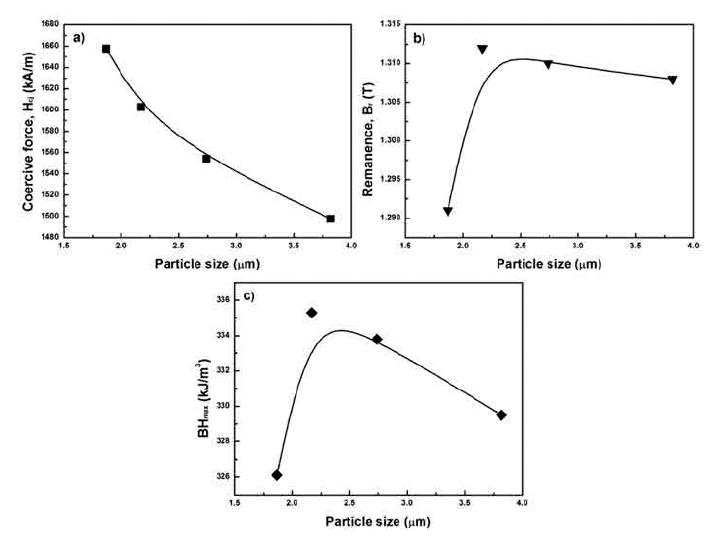
PDF Neodymium-iron-boron (Nd-Fe-B) sintered magnets have excellent magnetic properties such as the remanence, coercive force, and the maximum energy product compared to other hard magnetic materials. The coercive force of Nd-Fe-B sintered magnets is improved by the addition of heavy rare earth elements such as dysprosium and terbium instead of neodymium. Then, the magnetocrystalline anisotropy of Nd-Fe-B sintered magnets increases. However, additional elements have increased the production cost of Nd-Fe-B sintered magnets. Hence, a study on the control of the microstructure of Nd-Fe-B magnets is being conducted. As the coercive force of magnets improves, the grain size of the Nd2Fe14B grain is close to 300 nm because they are nucleation-type magnets. In this study, fine particles of Nd-Fe-B are prepared with various grinding energies in the pulverization process used for preparing sintered magnets, and the microstructure and magnetic properties of the magnets are investigated.
- [Korean]
- Synthesis and Characterization of Brilliant Yellow Color Pigments using α-FeOOH Nanorods
- JiYeon Yun, Ri Yu, YooJin Kim
- J Korean Powder Metall Inst. 2016;23(6):453-457. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.453

- 615 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this work, we synthesize brilliant yellow color α-FeOOH by controlling the rod length and core-shell structure. The characteristics of α-FeOOH nanorods are controlled by the reaction conditions. In particular, the length of the α-FeOOH rods depends on the concentration of the raw materials, such as the alkali solution. The length of the nanorods is adjusted from 68 nm to 1435 nm. Their yellowness gradually increases, with the highest b* value of 57 based on the International Commission on Illumination (CIE)
Lab system, by controlling the nanorod length. A high quality yellow color is obtained after formation of a silica coating on the α-FeOOH structure. The morphology and the coloration of the nal products are investigated in detail by X-ray diffraction, scanning electron microscopy, UV-vis spectroscopy, and the CIELab color parameter measurements.
- [Korean]
- Evaluation of TiO2 Photocatalytic Activity with Addition of Carbon Nanotube
- In-Chul Yeo, In-Cheol Kang
- J Korean Powder Metall Inst. 2016;23(6):458-465. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.458

- 520 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF A TiO2/CNT nanohybrid photocatalyst is synthesized via sol-gel route, with titanium (IV) isopropoxide and multi-walled carbon nanotubes (MWCNTs) as the starting materials. The microstructures and phase constitution of the nanohybrid TiO2/CNT (0.005wt%) samples after calcination at 450°C, 550°C and 650°C in air are compared with those of pure TiO2 using field-emission scanning electron microscopy and X-ray diffraction, respectively. In addition, the photocatalytic activity of the nanohybrid is compared with that of pure TiO2 with regard to the degradation of methyl orange under visible light irradiation. The TiO2/CNT composite exhibits a fast grain growth and phase transformation during calcination. The nanocomposite shows enhanced photocatalytic activity under visible light irradiation in comparison to pure TiO2 owing to not only better adsorption capability of CNT but also effective electron transfer between TiO2 and CNTs. However, the high calcination temperature of 650°C, regardless of addition of CNT, causes a decrease in photocatalytic activity because of grain growth and phase transformation to rutile. These results such as fast phase transformation to rutile and effective electron transfer are related to carbon doping into TiO2.
TOP
 KPMI
KPMI




 First
First Prev
Prev


