Search
- Page Path
- HOME > Search
- [Korean]
- The Synthesis of Lithium Lanthanum Titanium Oxide for Solid Electrolyte via Ultrasonic Spray Pyrolysis
- Jaeseok Roh, MinHo Yang, Kun-Jae Lee
- J Powder Mater. 2022;29(6):485-491. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.485

- 1,467 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF Lithium lanthanum titanium oxide (LLTO) is a promising ceramic electrolyte because of its high ionic conductivity at room temperature, low electrical conductivity, and outstanding physical properties. Several routes for the synthesis of bulk LLTO are known, in particular, solid-state synthesis and sol-gel method. However, the extremely low ionic conductivity of LLTO at grain boundaries is one of the major problems for practical applications. To diminish the grain boundary effect, the structure of LLTO is tuned to nanoscale morphology with structures of different dimensionalities (0D spheres, and 1D tubes and wires); this strategy has great potential to enhance the ion conduction by intensifying Li diffusion and minimizing the grain boundary resistance. Therefore, in this work, 0D spherical LLTO is synthesized using ultrasonic spray pyrolysis (USP). The USP method primarily yields spherical particles from the droplets generated by ultrasonic waves passed through several heating zones. LLTO is synthesized using USP, and the effects of each precursor and their mechanisms as well as synthesis parameters are analyzed and discussed to optimize the synthesis. The phase structure of the obtained materials is analyzed using X-ray diffraction, and their morphology and particle size are analyzed using field-emission scanning electron microscopy.
- [Korean]
- Pressureless Sintering and Microstructure of Pure Tungsten Powders Prepared by Ultrasonic Spray Pyrolysis
- Youn Ji Heo, Eui Seon Lee, Sung-Tag Oh, Jongmin Byun
- J Powder Mater. 2022;29(3):247-251. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.247
- 1,286 View
- 11 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
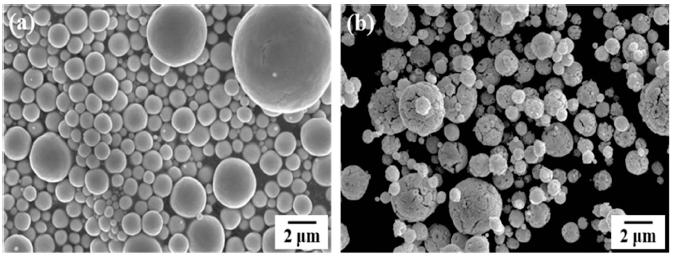
PDF This study demonstrates the effect of the compaction pressure on the microstructure and properties of pressureless-sintered W bodies. W powders are synthesized by ultrasonic spray pyrolysis and hydrogen reduction using ammonium metatungstate hydrate as a precursor. Microstructural investigation reveals that a spherical powder in the form of agglomerated nanosized W particles is successfully synthesized. The W powder synthesized by ultrasonic spray pyrolysis exhibits a relative density of approximately 94% regardless of the compaction pressure, whereas the commercial powder exhibits a relative density of 64% under the same sintering conditions. This change in the relative density of the sintered compact can be explained by the difference in the sizes of the raw powder and the densities of the compacted green body. The grain size increases as the compaction pressure increases, and the sintered compact uniaxially pressed to 50 MPa and then isostatically pressed to 300 MPa exhibits a size of 0.71 m. The Vickers hardness of the sintered W exhibits a high value of 4.7 GPa, mainly due to grain refinement.
-
Citations
Citations to this article as recorded by- Preparation of W-Ni-Cu Alloy Powder by Hydrogen Reduction of Metal Oxides
Youn Ji Heo, Eui Seon Lee, Ji Won Choi, Jongmin Byun, Sung-Tag Oh
Korean Journal of Metals and Materials.2024; 62(5): 334. CrossRef - Influence of the initial powder characteristic on the densified tungsten microstructure by spark plasma sintering and hot isostatic pressing
Ji Young Kim, Eui Seon Lee, Youn Ji Heo, Young-In Lee, Jongmin Byun, Sung-Tag Oh
Powder Metallurgy.2023; 66(5): 644. CrossRef
- Preparation of W-Ni-Cu Alloy Powder by Hydrogen Reduction of Metal Oxides
- [Korean]
- Synthesis and Optical Property of (GaN)1-x(ZnO)x Nanoparticles Using an Ultrasonic Spray Pyrolysis Process and Subsequent Chemical Transformation
- Jeong Hyun Kim, Cheol-Hui Ryu, Myungjun Ji, Yomin Choi, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(2):143-149. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.143
- 790 View
- 2 Download
-
 Abstract
Abstract
 PDF
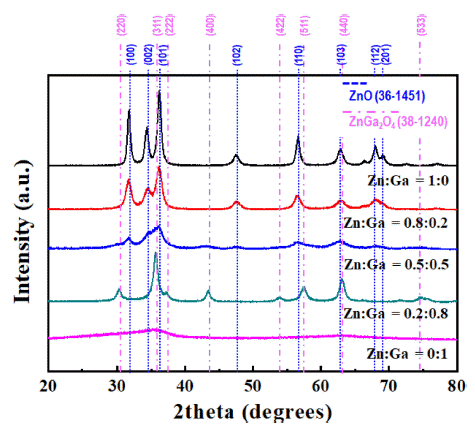
PDF In this study, (GaN)1-x(ZnO)x solid solution nanoparticles with a high zinc content are prepared by ultrasonic spray pyrolysis and subsequent nitridation. The structure and morphology of the samples are investigated by X-ray diffraction (XRD), field-emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The characterization results show a phase transition from the Zn and Ga-based oxides (ZnO or ZnGa2O4) to a (GaN)1-x (ZnO)x solid solution under an NH3 atmosphere. The effect of the precursor solution concentration and nitridation temperature on the final products are systematically investigated to obtain (GaN)1-x(ZnO)x nanoparticles with a high Zn concentration. It is confirmed that the powder synthesized from the solution in which the ratio of Zn and Ga was set to 0.8:0.2, as the initial precursor composition was composed of about 0.8-mole fraction of Zn, similar to the initially set one, through nitriding treatment at 700°C. Besides, the synthesized nanoparticles exhibited the typical XRD pattern of (GaN)1-x(ZnO)x, and a strong absorption of visible light with a bandgap energy of approximately 2.78 eV, confirming their potential use as a hydrogen production photocatalyst.
- [Korean]
- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Jae-Hyun Yoo, Myeong-Jun Ji, Woo-Young Park, Young-In Lee
- J Korean Powder Metall Inst. 2019;26(3):237-242. Published online June 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.3.237
- 837 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
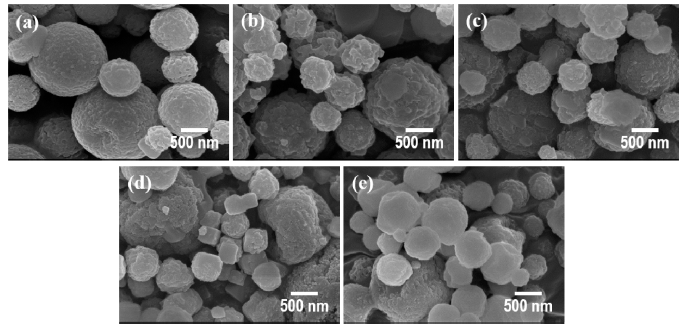
PDF In this study, ultrasonic spray pyrolysis combined with salt-assisted decomposition, a process that adds sodium nitrate (NaNO3) into a titanium precursor solution, is used to synthesize nanosized titanium dioxide (TiO2) particles. The added NaNO3 prevents the agglomeration of the primary nanoparticles in the pyrolysis process. The nanoparticles are obtained after a washing process, removing NaNO3 and NaF from the secondary particles, which consist of the salts and TiO2 nanoparticles. The effects of pyrolysis temperature on the size, crystallographic characteristics, and bandgap energy of the synthesized nanoparticles are systematically investigated. The synthesized TiO2 nanoparticles have a size of approximately 2–10 nm a bandgap energy of 3.1–3.25 eV, depending on the synthetic temperature. These differences in properties affect the photocatalytic activities of the synthesized TiO2 nanoparticles.
-
Citations
Citations to this article as recorded by- Microstructure and Sintering Behavior of Fine Tungsten Powders Synthesized by Ultrasonic Spray Pyrolysis
Hyeonhui Jo, Jeong Hyun Kim, Young-In Lee, Young-Keun Jeong, Sung-Tag Oh
Korean Journal of Metals and Materials.2021; 59(5): 289. CrossRef
- Microstructure and Sintering Behavior of Fine Tungsten Powders Synthesized by Ultrasonic Spray Pyrolysis
- [Korean]
- Investigation on Size Distribution of Tungsten-based Alloy Particles with Solvent Viscosity During Ultrasonic Ball Milling Process
- KeunHyuk Ryu, HyeongSub So, JiSeok Yun, InHo Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(3):201-207. Published online June 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.3.201
- 1,131 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
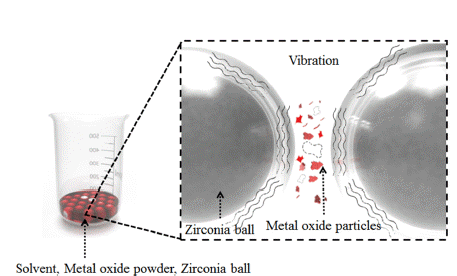
PDF Tungsten heavy alloys (W–Ni–Fe) play an important role in various industries because of their excellent mechanical properties, such as the excellent hardness of tungsten, low thermal expansion, corrosion resistance of nickel, and ductility of iron. In tungsten heavy alloys, tungsten nanoparticles allow the relatively low-temperature molding of high-melting-point tungsten and can improve densification. In this study, to improve the densification of tungsten heavy alloy, nanoparticles are manufactured by ultrasonic milling of metal oxide. The physical properties of the metal oxide and the solvent viscosity are selected as the main parameters. When the density is low and the Mohs hardness is high, the particle size distribution is relatively high. When the density is high and the Mohs hardness is low, the particle size distribution is relatively low. Additionally, the average particle size tends to decrease with increasing viscosity. Metal oxides prepared by ultrasonic milling in high-viscosity solvent show an average particle size of less than 300 nm based on the dynamic light scattering and scanning electron microscopy analysis. The effects of the physical properties of the metal oxide and the solvent viscosity on the pulverization are analyzed experimentally.
-
Citations
Citations to this article as recorded by- Morphological Control and Surface Modification Characteristics of Nickel Oxalate Synthesized via Oxalic Acid Precipitation
Eunbi Park, Jongwon Bae, Sera Kang, Minsu Kang, Suseong Lee, Kun-Jae Lee
Journal of Powder Materials.2025; 32(5): 375. CrossRef - Manufacture of high sensitive Ag-Fe3O4-PDMS nanocomposite pressure sensor through morphology control of conductive filler
Keunhyuk Ryu, Namhun Kwon, Kun-Jae Lee
Advanced Powder Technology.2021; 32(7): 2441. CrossRef - Grinding behavior of WO3, NiO, Fe2O3 by ultrasonic milling parameters control and preparation of nanocomposite powder
Keunhyuk Ryu, Kun-Jae Lee
Advanced Powder Technology.2020; 31(9): 3867. CrossRef
- Morphological Control and Surface Modification Characteristics of Nickel Oxalate Synthesized via Oxalic Acid Precipitation
- [Korean]
- Synthesis and Optical Property of TiO2 Nanoparticles Using a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Myeong-Jun Ji, Woo-Young Park, Jae-Hyun Yoo, Young-In Lee
- J Korean Powder Metall Inst. 2019;26(1):34-39. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.34
- 835 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Current synthesis processes for titanium dioxide (TiO2) nanoparticles require expensive precursors or templates as well as complex steps and long reaction times. In addition, these processes produce highly agglomerated nanoparticles. In this study, we demonstrate a simple and continuous approach to synthesize TiO2 nanoparticles by a salt-assisted ultrasonic spray pyrolysis method. We also investigate the effect of salt content in a precursor solution on the morphology and size of synthesized products. The synthesized TiO2 nanoparticles are systematically characterized by X-ray diffraction, transmission electron micrograph, and UV-Vis spectroscopy. These nanoparticles appear to have a single anatase phase and a uniform particle-size distribution with an average particle size of approximately 10 nm. By extrapolating the plots of the transformed Kubelka-Munk function versus the absorbed light energy, we determine that the energy band gap of the synthesized TiO2 nanoparticles is 3.25 eV.
-
Citations
Citations to this article as recorded by- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
Jae-Hyun Yoo, Myeong-Jun Ji, Woo-Young Park, Young-In Lee
Journal of Korean Powder Metallurgy Institute.2019; 26(3): 237. CrossRef
- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
- [Korean]
- Synthesis and Optical Property of GaN Powder Using an Ultrasonic Spray Pyrolysis Process and Subsequent Nitridation Treatment
- Myeong-Jun Ji, Jae-Hyun Yoo, Young-In Lee
- J Korean Powder Metall Inst. 2018;25(6):482-486. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.482

- 765 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Despite numerous advances in the preparation and use of GaN, and many leading-edge applications in lighting technologies, the preparation of high-quality GaN powder remains a challenge. Ammonolytic preparations of polycrystalline GaN have been studied using various precursors, but all were time-consuming and required high temperatures. In this study, an efficient and low-temperature method to synthesize high-purity hexagonal GaN powder is developed using sub-micron Ga2O3 powder as a starting material. The sub-micron Ga2O3 powder was prepared by an ultrasonic spray pyrolysis process. The GaN powder is synthesized from the sub-micron Ga2O3 powder through a nitridation treatment in an NH3 flow at 800°C. The characteristics of the synthesized powder are systematically examined by X-ray diffraction, scanning and transmission electron microscopy, and UV-vis spectrophotometer.
- [Korean]
- Fabrication of compact surface structure by molar concentration on Sb-doped SnO2 transparent conducting films
- Ju-Won Bae, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2018;25(1):54-59. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.54
- 735 View
- 2 Download
-
 Abstract
Abstract
 PDF
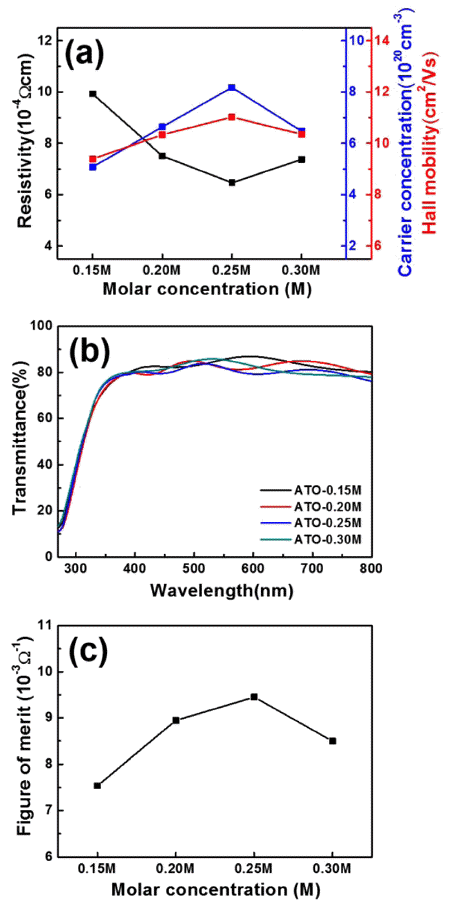
PDF Sb-doped SnO2 (ATO) transparent conducting films are fabricated using horizontal ultrasonic spray pyrolysis deposition (HUSPD) to form uniform and compact film structures with homogeneously supplied precursor solution. To optimize the molar concentration and transparent conducting performance of the ATO films using HUSPD, we use precursor solutions of 0.15, 0.20, 0.25, and 0.30 M. As the molar concentration increases, the resultant ATO films exhibit more compact surface structures because of the larger crystallite sizes and higher ATO crystallinity because of the greater thickness from the accelerated growth of ATO. Thus, the ATO films prepared at 0.25 M have the best transparent conducting performance (12.60±0.21 Ω/□ sheet resistance and 80.83% optical transmittance) and the highest figure-of-merit value (9.44±0.17 × 10-3 Ω-1). The improvement in transparent conducting performance is attributed to the enhanced carrier concentration by the improved ATO crystallinity and Hall mobility with the compact surface structure and preferred (211) orientation, ascribed to the accelerated growth of ATO at the optimized molar concentration. Therefore, ATO films fabricated using HUSPD are transparent conducting film candidates for optoelectronic devices.
- [Korean]
- Fabrication of Uniform TiO2 Blocking Layers for Prevention of Electron Recombination in Dye-Sensitized Solar Cells
- Ju-won Bae, Bon-Ryul Koo, Tae-Kuen Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2018;25(1):1-6. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.1
- 756 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
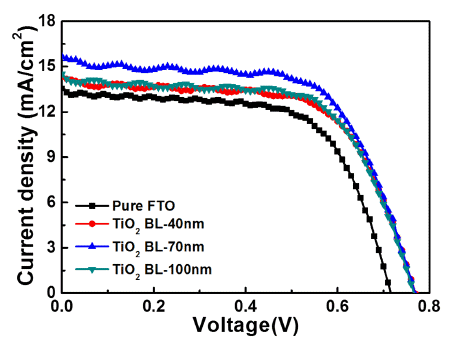
PDF Uniform TiO2 blocking layers (BLs) are fabricated using ultrasonic spray pyrolysis deposition (USPD) method. To improve the photovoltaic performance of dye-sensitized solar cells (DSSCs), the BL thickness is controlled by using USPD times of 0, 20, 60, and 100 min, creating TiO2 BLs of 0, 40, 70, and 100 nm, respectively, in average thickness on fluorine-doped tin oxide (FTO) glass. Compared to the other samples, the DSSC containing the uniform TiO2 BL of 70 nm in thickness shows a superior power conversion efficiency of 7.58±0.20% because of the suppression of electron recombination by the effect of the optimized thickness. The performance improvement is mainly attributed to the increased open-circuit voltage (0.77±0.02 V) achieved by the increased Fermi energy levels of the working electrodes and the improved short-circuit current density (15.67±0.43 mA/cm2) by efficient electron transfer pathways. Therefore, optimized TiO2 BLs fabricated by USPD may allow performance improvements in DSSCs.
-
Citations
Citations to this article as recorded by- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
Seung Hwan Ji, Hyunsu Park, Doyeon Kim, Do Hyung Han, Hye Won Yun, Woo-Byoung Kim
Korean Journal of Materials Research.2019; 29(3): 183. CrossRef - Surface tailoring of zinc electrodes for energy storage devices with high-energy densities and long cycle life
Geon-Hyoung An, SeungNam Cha, Jung Inn Sohn
Applied Surface Science.2019; 467-468: 1157. CrossRef - Crystallinity Control Effects on Vanadium Oxide Films for Enhanced Electrochromic Performances
Kue-Ho Kim, Ju-Won Bae, Tae-Kuen Lee, Hyo-Jin Ahn
Korean Journal of Materials Research.2019; 29(6): 385. CrossRef
- Flexible Dye-sensitized Solar Cell Using Titanium Gel at Low Temperature
- [Korean]
- Synthesis and Optical Property of BaTiO3 Nanoparticles Using a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Young Hwangbo, Young-In Lee
- J Korean Powder Metall Inst. 2017;24(4):326-331. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.326
- 904 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
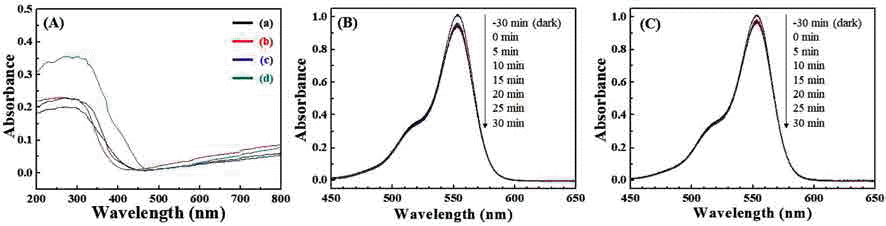
PDF The structural formation of inorganic nanoparticles dispersed in polymer matrices is a key technology for producing advanced nanocomposites with a unique combination of optical, electrical, and mechanical properties. Barium titanate (BaTiO3) nanoparticles are attractive for increasing the refractive index and dielectric constant of polymer nanocomposites. Current synthesis processes for BaTiO3 nanoparticles require expensive precursors or organic solvents, complicated steps, and long reaction times. In this study, we demonstrate a simple and continuous approach for synthesizing BaTiO3 nanoparticles based on a salt-assisted ultrasonic spray pyrolysis method. This process allows the synthesis of BaTiO3 nanoparticles with diameters of 20-50 nm and a highly crystalline tetragonal structure. The optical properties and photocatalytic activities of the nanoparticles show that they are suitable for use as fillers in various nanocomposites.
-
Citations
Citations to this article as recorded by- Sr doping effects on La(1-x)SrxMnO3-BaTiO3 nanocomposites: A comprehensive analysis of structural, optical, magnetic, and dielectric properties
Milad Karamzadeh-Jahromi, Morteza Izadifard, Mohammad Ebrahim Ghazi
Journal of Alloys and Compounds.2024; 1006: 176272. CrossRef
- Sr doping effects on La(1-x)SrxMnO3-BaTiO3 nanocomposites: A comprehensive analysis of structural, optical, magnetic, and dielectric properties
- [English]
- Dispersion Behavior and Size Analysis of Thermally Purified High Pressure-high Temperature Synthesized Nanodiamond Particles
- Hansang Kwon, Jehong Park, Marc Leparoux
- J Korean Powder Metall Inst. 2017;24(3):216-222. Published online June 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.3.216

- 1,384 View
- 4 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Synthesized monocrystalline nanodiamond (nD) particles are heat-treated at various temperatures to produce highly structured diamond crystals. The heat-treated nDs show different weight loss ratios during thermogravimetric analysis. The crystallinities of the heat-treated nDs are analyzed using Raman spectroscopy. The average particle sizes of the heat-treated nDs are measured by a dynamic light scattering (DLS) system and direct imaging observation methods. Moreover, individual dispersion behaviors of the heat-treated nD particles are investigated based on ultrasonic dispersion methods. The average particle sizes of the dispersed nDs according to the two different measurement methods show very similar size distributions. Thus, it is possible to produce highly crystallized nD powder particles by a heattreatment process, and the nD particles are relatively easy to disperse individually without any dispersant. The heattreated nDs can lead to potential applications such as in nanocomposites, quantum dots, and biomedical materials.
-
Citations
Citations to this article as recorded by- Two extreme crystal size scales of diamonds, large single crystal and nanocrystal diamonds: Synthesis, properties and their mutual transformation
Yang Wang, Wei-hua Wang, Shi-lin Yang, Guo-yang Shu, Bing Dai, Jia-qi Zhu
New Carbon Materials.2021; 36(3): 512. CrossRef
- Two extreme crystal size scales of diamonds, large single crystal and nanocrystal diamonds: Synthesis, properties and their mutual transformation
- [Korean]
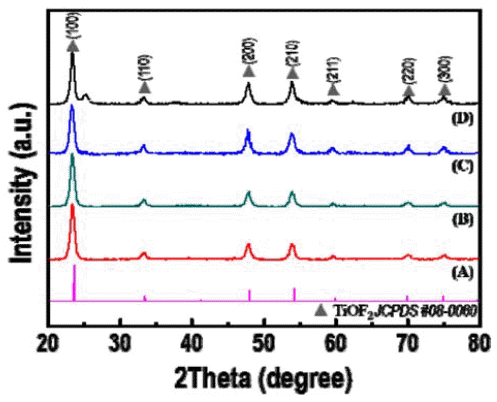
- Synthesis and Optical Property of a TiOF2 Powder via an Ultrasonic Spray Pyrolysis Process
- Young Hwangbo, Young-In Lee
- J Korean Powder Metall Inst. 2016;23(4):307-310. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.307
- 681 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF TiOF2, which has remarkable electrochemical and optical properties, is used in various applications such as Li-ion batteries, electrochemical displays, and photocatalysts. In addition, it is possible to utilize the template which is allowed to synthesize fluorine doped TiO2 powders with hollow or faceted structures. However, common synthesis methods of TiOF2 powders have some disadvantages such as the use of expensive and harmful precursors and batchtype processes with a limited production scale. In this study, we report a synthetic route for preparing TiOF2 powders by using an inexpensive and harmless precursor and a continuous ultrasonic spray pyrolysis process under a controlled atmosphere to address the aforementioned problems. The synthesized powder has an average size of 1 μm, a spherical shape, a pure TiOF2 phase, and exhibits a band-gap energy of 3.2 eV.
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- [Korean]
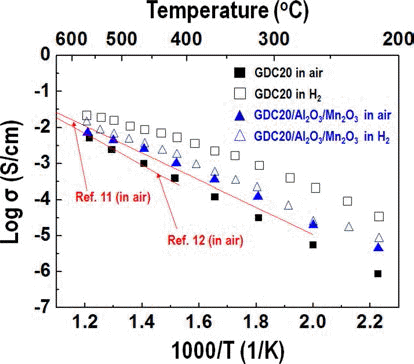
- Synthesis and Characterization of a Ceria Based Composite Electrolyte for Solid Oxide Fuel Cells by an Ultrasonic Spray Pyrolysis Process
- Young-In Lee, Yong-Ho Choa
- J Korean Powder Metall Inst. 2014;21(3):222-228. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.222
- 887 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Much research into fuel cells operating at a temperature below 800°C. is being performed. There are significant efforts to replace the yttria-stabilized zirconia electrolyte with a doped ceria electrolyte that has high ionic conductivity even at a lower temperature. Even if the doped ceria electrolyte has high ionic conductivity, it also shows high electronic conductivity in a reducing environment, therefore, when used as a solid electrolyte of a fuel cell, the powergeneration efficiency and mechanical properties of the fuel cell may be degraded. In this study, gadolinium-doped ceria nanopowder with Al2O3 and Mn2O3 as a reinforcing and electron trapping agents were synthesized by ultrasonic pyrolysis process. After firing, their microstructure and mechanical and electrical properties were investigated and compared with those of pure gadolinium-doped ceria specimen.
-
Citations
Citations to this article as recorded by- High growth-rate atomic layer deposition process of cerium oxide thin film for solid oxide fuel cell
Jin-Geun Yu, Byung Chan Yang, Jeong Woo Shin, Sungje Lee, Seongkook Oh, Jae-Ho Choi, Jaehack Jeong, Wontae Noh, Jihwan An
Ceramics International.2019; 45(3): 3811. CrossRef - Atomic-layer-deposited ZrO2-doped CeO2 thin film for facilitating oxygen reduction reaction in solid oxide fuel cell
Byung Chan Yang, Dohyun Go, Seongkook Oh, Jeong Woo Shin, Hyong June Kim, Jihwan An
Applied Surface Science.2019; 473: 102. CrossRef
- High growth-rate atomic layer deposition process of cerium oxide thin film for solid oxide fuel cell
TOP
 KPMI
KPMI


 First
First Prev
Prev


