Search
- Page Path
- HOME > Search
- [Korean]
- Enhanced H2S Gas Sensing Using ZnO Porous Nanorod Synthesized via a Rotational Hydrothermal Method
- Jimyeong Park, Changyu Kim, Minseo Kim, Jiyeon Shin, Jae-Hyoung Lee, Myung Sik Choi
- J Powder Mater. 2025;32(5):406-415. Published online October 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00262
- 431 View
- 9 Download
-
 Abstract
Abstract
 PDF
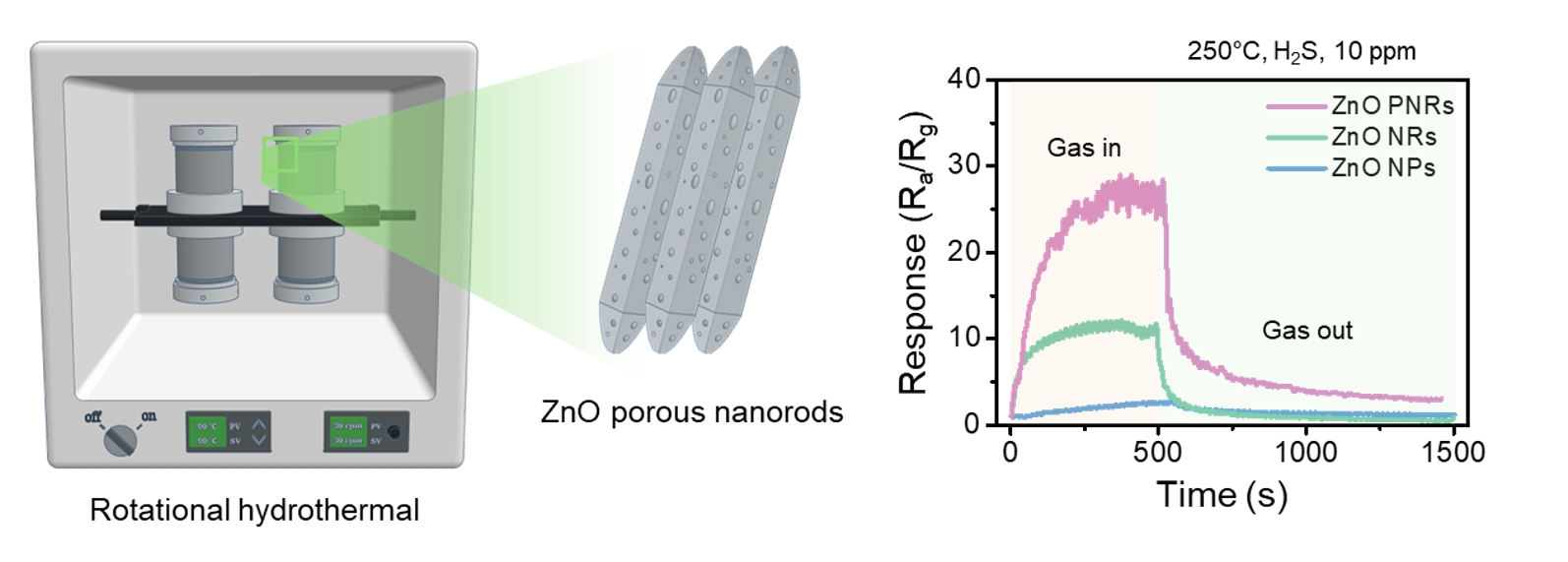
PDF - In this study, ZnO porous nanorods were synthesised using a rotational hydrothermal process, and their performance as hydrogen sulphide (H2S) gas sensors was analysed. Compared to commercial ZnO nanoparticles and conventionally hydrothermally synthesised ZnO nanorods, the ZnO porous nanorods exhibited a more uniform structure and improved crystal growth in the (002) plane, with surfaces rich in porosity and oxygen vacancies. These structural and chemical characteristics significantly improved the sensitivity toward H2S, showing high detection performance at 250°C across various concentrations of H2S gas. Additionally, the sensor demonstrated excellent selectivity against other gases such as C2H5OH, C6H6, C7H8, and NH3. This study indicated that the rotational hydrothermal process is an effective method for developing high-performance ZnO-based gas sensors and suggests its applicability to other metal oxide materials.
- [Korean]
- Aqueous Synthesis and Luminescent Characteristics of Cu:ZnSe Quantum Dots by Internal Doping Method
- Geum Ji Back, Hyun Seon Hong
- J Powder Mater. 2022;29(5):370-375. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.370
- 862 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
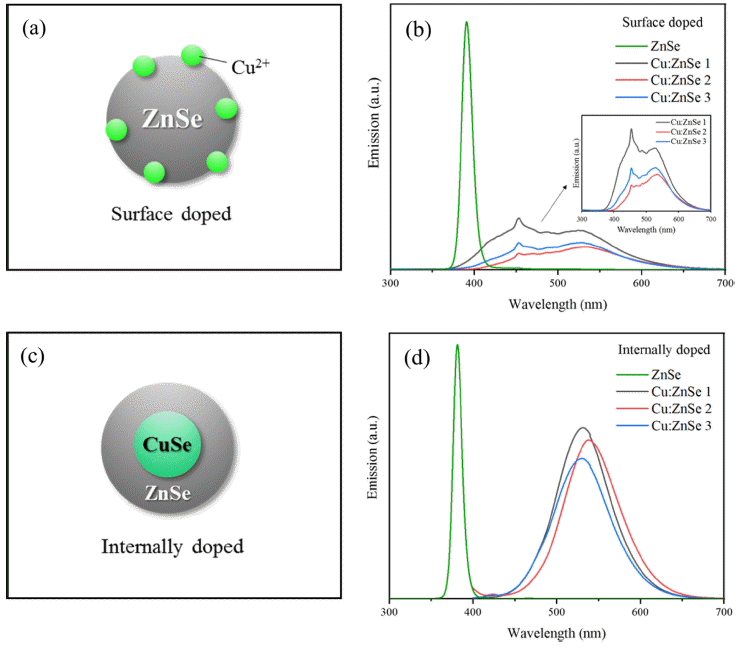
PDF Cu-doped ZnSe quantum dots were successfully synthesized in an aqueous solution using an internal doping method. The effects of ligand type, CuSe synthesis temperature, and heating time on Cu-doped ZnSe synthesis were systematically investigated. Of MPA, GSH, TGA, and NAC used as ligands, MPA was the optimal ligand as determined by PL spectrum analysis. In addition, the emission wavelength was found to depend on the synthesis temperature of the internal doping core of CuSe. As the temperature increased, the doping of Cu2+ was enhanced, and the emission wavelength band was redshifted; accordingly, the emission peaks moved from blue to green (up to 550 nm). Thus, the synthesis of Cu:ZnSe using internal doping in aqueous solutions is a potential method for ecomanufacturing of colortuned ZnSe quantum dots for display applications.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
- [Korean]
- A Study on Morphology Control of (Ga1-xZnx)(N1-xOx) Nanofibers according to the Composition and Crystallinity of Oxide Nanofibers Synthesized by Electrospinning
- Jeong Hyun Kim, Sung-Tag Oh, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(3):259-266. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.259
- 653 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
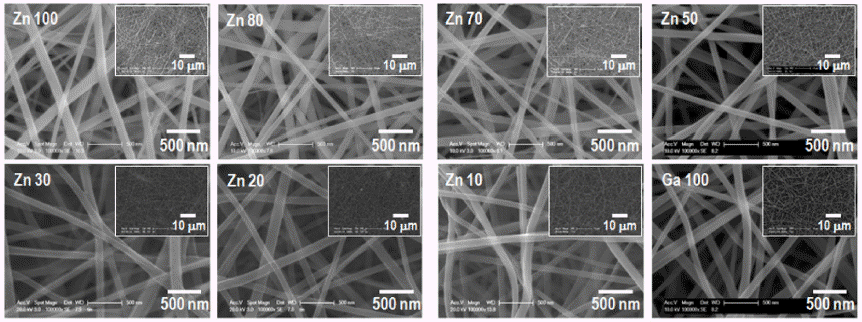
PDF The (Ga1-xZnx)(N1-xOx) solid solution is attracting extensive attention for photocatalytic water splitting and wastewater treatment owing to its narrow and controllable band gap. To optimize the photocatalytic performance of the solid solution, the key points are to decrease its band gap and recombination rate. In this study, (Ga1-xZnx)(N1-xOx) nanofibers with various Zn fractions are prepared by electrospinning followed by calcination and nitridation. The effect of the composition and crystallinity of electrospun oxide nanofibers on the morphology and optical properties of the obtained solid-solution nanofibers are systematically investigated. The results show that the final shape of the (Ga1-xZnx) (N1-xOx) material is greatly affected by the crystallinity of the oxide nanofibers before nitridation. The photocatalytic properties of (Ga1-xZnx)(N1-xOx) with different Ga:Zn atomic ratios are investigated by studying the degradation of rhodamine B under visible light irradiation.
-
Citations
Citations to this article as recorded by- Fabrication of Nanowire by Electrospinning Process Using Nickel Oxide Particle Recovered from MLCC
Haein Shin, Jongwon Bae, Minsu Kang, Kun-Jae Lee
journal of Korean Powder Metallurgy Institute.2023; 30(6): 502. CrossRef
- Fabrication of Nanowire by Electrospinning Process Using Nickel Oxide Particle Recovered from MLCC
- [Korean]
- Rotation Speed Dependence of ZnO Coating Layer on SnSe powders by Rotary Atomic Layer Deposition Reactor
- Myeong Jun Jung, Ye Jun Yun, Jongmin Byun, Byung Joon Choi
- J Korean Powder Metall Inst. 2021;28(3):239-245. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.239
- 638 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The SnSe single crystal shows an outstanding figure of merit (
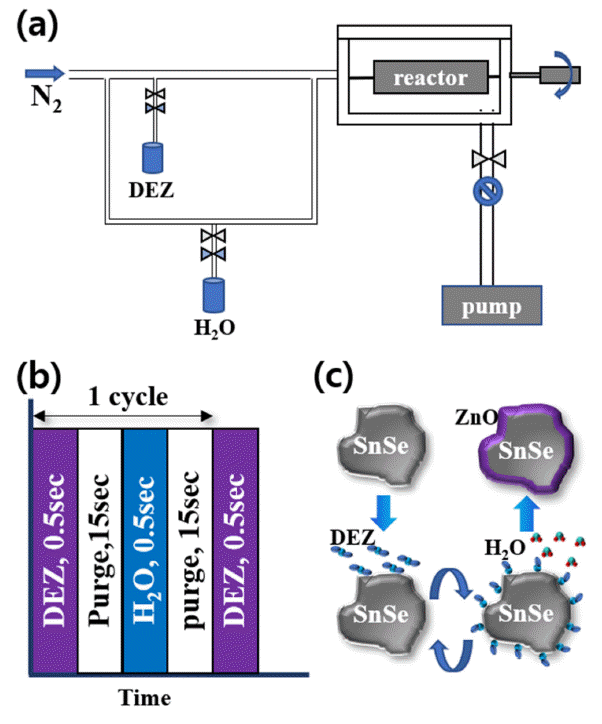
ZT ) of 2.6 at 973 K; thus, it is considered to be a promising thermoelectric material. However, the mass production of SnSe single crystals is difficult, and their mechanical properties are poor. Alternatively, we can use polycrystalline SnSe powder, which has better mechanical properties. In this study, surface modification by atomic layer deposition (ALD) is chosen to increase theZT value of SnSe polycrystalline powder. SnSe powder is ground by a ball mill. An ALD coating process using a rotary-type reactor is adopted. ZnO thin films are grown by 100 ALD cycles using diethylzinc and H2O as precursors at 100°C. ALD is performed at rotation speeds of 30, 40, 50, and 60 rpm to examine the effects of rotation speed on the thin film characteristics. The physical and chemical properties of ALD-coated SnSe powders are characterized by scanning and tunneling electron microscopy combined with energy-dispersive spectroscopy. The results reveal that a smooth oxygenrich ZnO layer is grown on SnSe at a rotation speed of 30 rpm. This result can be applied for the uniform coating of a ZnO layer on various powder materials.-
Citations
Citations to this article as recorded by- Thermal Confinement and Filtering Effect of SnSe by Insertion of Atomic-Layer-Deposited ZnO Interfacial Layer
Myeong Jun Jung, Su Min Eun, Hogyoung Kim, Seong Keun Kim, Jongmin Byun, Byung Joon Choi
Korean Journal of Chemical Engineering.2025; 42(14): 3545. CrossRef
- Thermal Confinement and Filtering Effect of SnSe by Insertion of Atomic-Layer-Deposited ZnO Interfacial Layer
- [Korean]
- Synthesis and Optical Property of (GaN)1-x(ZnO)x Nanoparticles Using an Ultrasonic Spray Pyrolysis Process and Subsequent Chemical Transformation
- Jeong Hyun Kim, Cheol-Hui Ryu, Myungjun Ji, Yomin Choi, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(2):143-149. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.143
- 790 View
- 2 Download
-
 Abstract
Abstract
 PDF
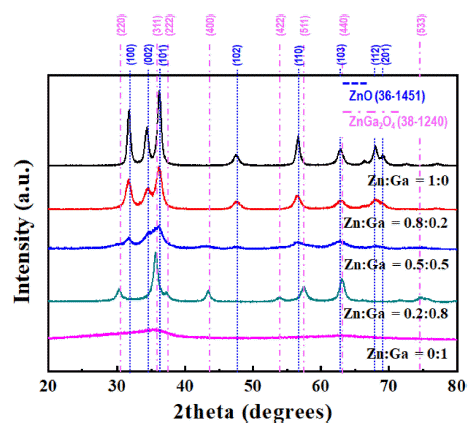
PDF In this study, (GaN)1-x(ZnO)x solid solution nanoparticles with a high zinc content are prepared by ultrasonic spray pyrolysis and subsequent nitridation. The structure and morphology of the samples are investigated by X-ray diffraction (XRD), field-emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The characterization results show a phase transition from the Zn and Ga-based oxides (ZnO or ZnGa2O4) to a (GaN)1-x (ZnO)x solid solution under an NH3 atmosphere. The effect of the precursor solution concentration and nitridation temperature on the final products are systematically investigated to obtain (GaN)1-x(ZnO)x nanoparticles with a high Zn concentration. It is confirmed that the powder synthesized from the solution in which the ratio of Zn and Ga was set to 0.8:0.2, as the initial precursor composition was composed of about 0.8-mole fraction of Zn, similar to the initially set one, through nitriding treatment at 700°C. Besides, the synthesized nanoparticles exhibited the typical XRD pattern of (GaN)1-x(ZnO)x, and a strong absorption of visible light with a bandgap energy of approximately 2.78 eV, confirming their potential use as a hydrogen production photocatalyst.
- [Korean]
- Effects of Synthesis Conditions on Luminescence Characteristics of Glutathione Capped ZnSe Nano particles
- Geum Ji Back, Ha Yeon Song, Min Seo Lee, Hyun Seon Hong
- J Korean Powder Metall Inst. 2021;28(1):44-50. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.44
- 1,205 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
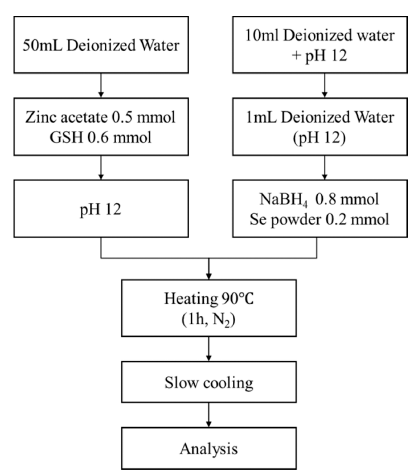
PDF Zinc selenide (ZnSe) nanoparticles were synthesized in aqueous solution using glutathione (GSH) as a ligand. The influence of the ligand content, reaction temperature, and hydroxyl ion concentration (pH) on the fabrication of the ZnSe particles was investigated. The optical properties of the synthesized ZnSe particles were characterized using various analytical techniques. The nanoparticles absorbed UV-vis light in the range of 350-400 nm, which is shorter than the absorption wavelength of bulk ZnSe particles (460 nm). The lowest ligand concentration for achieving good light absorption and emission properties was 0.6 mmol. The reaction temperature had an impact on the emission properties; photoluminescence spectroscopic analysis showed that the photo-discharge characteristics were greatly enhanced at high temperatures. These discharge characteristics were also affected by the hydroxyl ion concentration in solution; at pH 13, sound emission characteristics were observed, even at a low temperature of 25°C. The manufactured nanoparticles showed excellent light absorption and emission properties, suggesting the possibility of fabricating ZnSe QDs in aqueous solutions at low temperatures.
-
Citations
Citations to this article as recorded by- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
Geum Ji Back, Ha Yeon Song, Min Seo Lee, Jaesik Yoon, Hyun Seon Hong
Journal of Crystal Growth.2024; 626: 127475. CrossRef - Effect of UV Irradiation on Optical Properties of Water-Based Synthetic Zinc Selenide Quantum Dots
Geum Ji Back, Yu Jin Kang, I Ju Kang, Jeong Hyeon Lim, Hyun Seon Hong
Korean Journal of Metals and Materials.2022; 60(2): 160. CrossRef
- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
- [Korean]
- Influence of Reducing Agents and Additives on the Synthesis of ZnSe Nanoparticles
- Geum Ji Back, Da Gyeong Lee, Min Seo Lee, Ha Yeon Song, Hyun Seon Hong
- J Korean Powder Metall Inst. 2020;27(3):233-240. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.233
- 630 View
- 1 Download
-
 Abstract
Abstract
 PDF
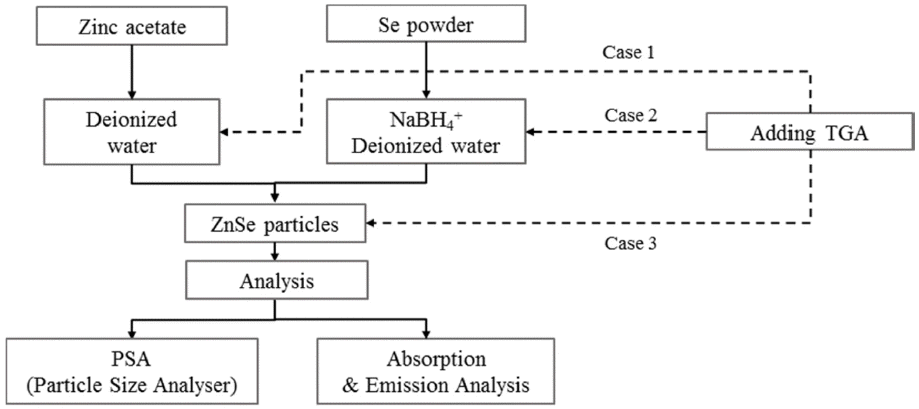
PDF Nano-sized ZnSe particles are successfully synthesized in an aqueous solution at room temperature using sodium borohydride (NaBH4) and thioglycolic acid (TGA) as the reducing agent and stabilizer, respectively. The effects of the mass ratio of the reducing agent to Se, stabilizer concentration, and stirring time on the synthesis of the ZnSe nanoparticles are evaluated. The light absorption/emission properties of the synthesized nanoparticles are characterized using ultraviolet-visible (UV-vis) spectroscopy, photoluminescence (PL) spectroscopy, and particle size analyzer (PSA) techniques. At least one mass ratio (NaBH4/Se) of the reducing agent should be added to produce ZnSe nanoparticles finer than 10 nm and to absorb UV–vis light shorter than the ZnSe bulk absorption wavelength of 460 nm. As the ratio of the reducing agent increases, the absorption wavelengths in the UV-vis curves are blue-shifted. Stirring in the atmosphere acts as a deterrent to the reduction reaction and formation of nanoparticles, but if not stirred in the atmosphere, the result is on par with synthesis in a nitrogen atmosphere. The stabilizer, TGA, has an impact on the Zn precursor synthesis. The fabricated nanoparticles exhibit excellent photo-absorption/discharge characteristics, suggesting that ZnSe nanoparticles can be alloyed without the need for organic solutions or high-temperature environments.
- [Korean]
- Synthesis and analysis CdSe/ZnS quantum dot with a Core/shell Continuous Synthesis System Using a Microfluidic Reactor
- Myung Hwan Hong, So Young Joo, Lee-Seung Kang, Chan Gi Lee
- J Korean Powder Metall Inst. 2018;25(2):132-136. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.132
- 1,932 View
- 10 Download
-
 Abstract
Abstract
 PDF
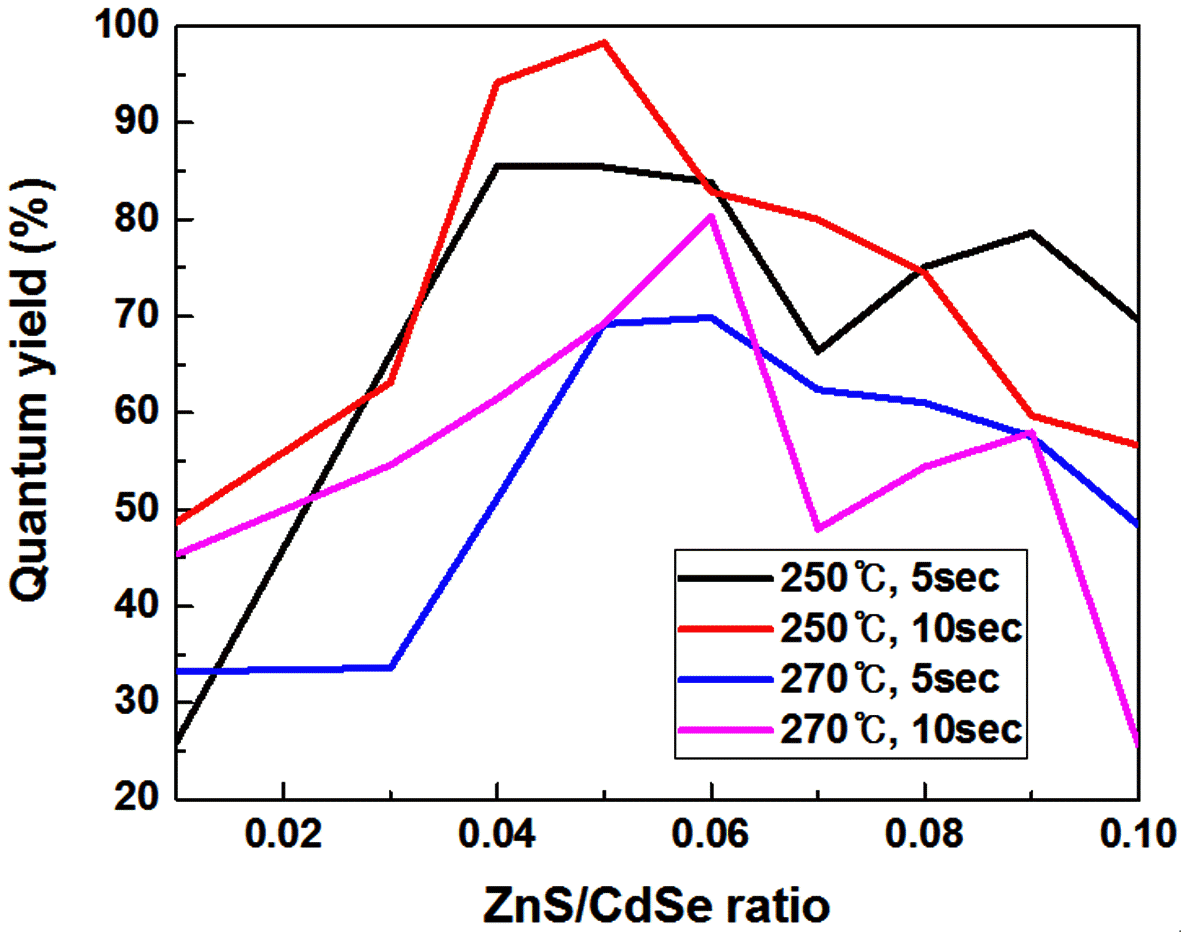
PDF Core/shell CdSe/ZnS quantum dots (QDs) are synthesized by a microfluidic reactor-assisted continuous reactor system. Photoluminescence and absorbance of synthesized CdSe/ZnS core/shell QDs are investigated by fluorescence spectrophotometry and online UV-Vis spectrometry. Three reaction conditions, namely; the shell coating reaction temperature, the shell coating reaction time, and the ZnS/CdSe precursor volume ratio, are combined in the synthesis process. The quantum yield of the synthesized CdSe QDs is determined for each condition. CdSe/ZnS QDs with a higher quantum yield are obtained compared to the discontinuous microfluidic reactor synthesis system. The maximum quantum efficiency is 98.3% when the reaction temperature, reaction time, and ZnS/CdSe ratio are 270°C, 10 s, and 0.05, respectively. Obtained results indicate that a continuous synthesis of the Core/shell CdSe/ZnS QDs with a high quantum efficiency could be achieved by isolating the reaction from the external environment.
- [Korean]
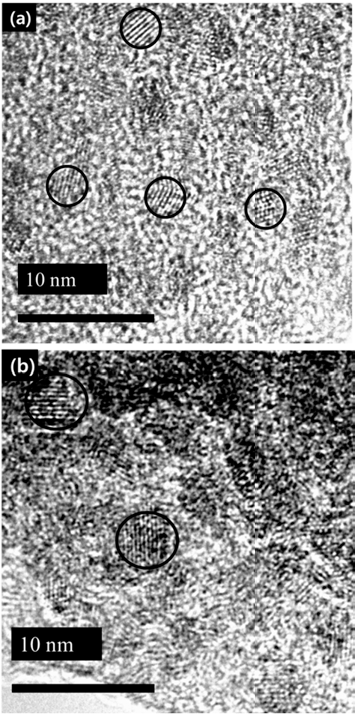
- Luminescence Properties of InP/ZnS Quantum Dots depending on InP Core synthesis Temperature
- Han Wook Seo, Da-Woon Jeong, Min Young Kim, Seoung Kyun Hyun, Ji Sun On, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(4):321-325. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.321
- 1,476 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, we investigate the optical properties of InP/ZnS core/shell quantum dots (QDs) by controlling the synthesis temperature of InP. The size of InP determined by the empirical formula tends to increase with temperature: the size of InP synthesized at 140oC and 220oC is 2.46 nm and 4.52 nm, respectively. However, the photoluminescence (PL) spectrum of InP is not observed because of the formation of defects on the InP surface. The growth of InP is observed during the deposition of the shell (ZnS) on the synthesized InP, which is ended up with green-red PL spectrum. We can adjust the PL spectrum and absorption spectrum of InP/ZnS by simply adjusting the core temperature. Thus, we conclude that there exists an optimum shell thickness for the QDs according to the size.
-
Citations
Citations to this article as recorded by- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- [Korean]
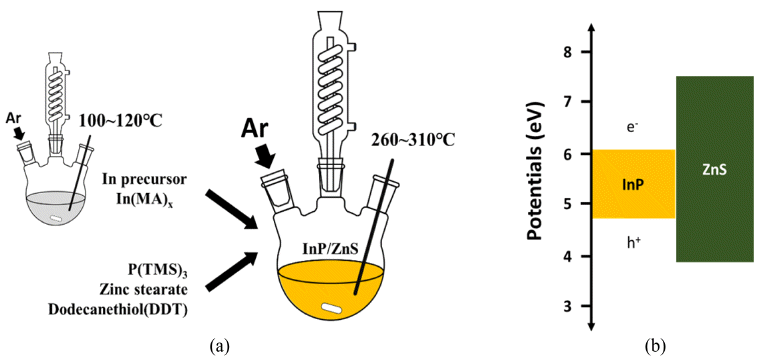
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,037 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
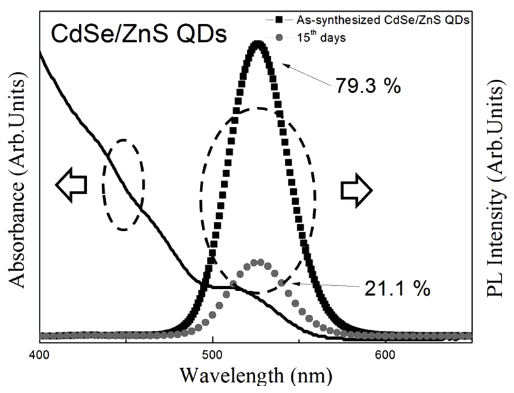
- Surface Treatment Method for Long-term Stability of CdSe/ZnS Quantum Dots
- Hyun-Su Park, Da-Woon Jeong, Bum-Sung Kim, So-Yeong Joo, Chan-Gi Lee, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(1):1-5. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.1
- 1,541 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF We have investigated the washing method of as-synthesized CdSe/ZnS core/shell structure quantum dots (QDs) and the effective surface passivation method of the washed QDs using PMMA. The quantum yield (QY%) of assynthesized QDs decreases with time, from 79.3% to 21.1%, owing to surface reaction with residual organics. The decreased QY% is restored to the QY% of as-synthesized QDs by washing. However, the QY% of washed QDs also decreases with time, owing to the absence of surface passivation layer. On the other hand, the PMMA-treated QDs maintained a relatively higher QY% after washing than that of the washed QDs that were kept in toluene solution for 30 days. Formation of the PMMA coating layer on CdSe/ZnS QD surface is confirmed by HR-TEM and FT-IR. It is found that the PMMA surface coating, when combined with washing, is useful to be applied in the storage of QDs, owing to its long-term stability.
-
Citations
Citations to this article as recorded by- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
Seung Hwan Ji, Hye Won Yun, Jin Ho Lee, Bum-Sung Kim, Woo-Byoung Kim
Korean Journal of Materials Research.2021; 31(1): 16. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
- [Korean]
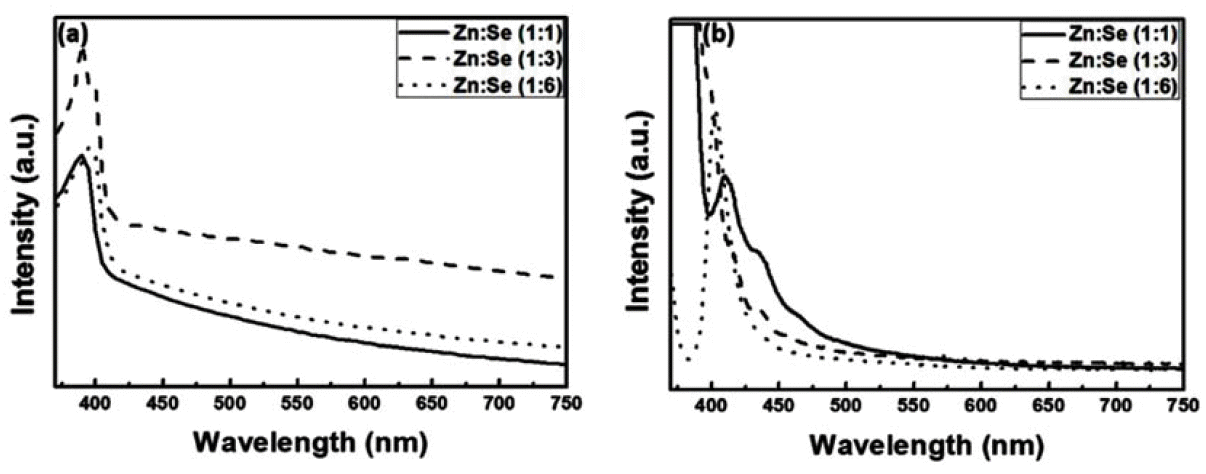
- The Effect of Surface Defects on the Optical Properties of ZnSe:Eu Quantum Dots
- Da-Woon Jeong, Ji Young Park, Han Wook Seo, Kyoung-Mook Lim, Tae-Yeon Seong, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(5):348-352. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.348
- 1,323 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Quantum dots (QDs) are capable of controlling the typical emission and absorption wavelengths because of the bandgap widening effect of nanometer-sized particles. These phosphor particles have been used in optical devices, photovoltaic devices, advanced display devices, and several biomedical complexes. In this study, we synthesize ZnSe QDs with controlled surface defects by a heating-up method. The optical properties of the synthesized particles are analyzed using UV-visible and photoluminescence (PL) measurements. Calculations indicate nearly monodisperse particles with a size of about 5.1 nm at 260°C (full width at half maximum = 27.7 nm). Furthermore, the study results confirm that successful doping is achieved by adding Eu3+ preparing the growth phase of the ZnSe:Eu QDs when heating-up method. Further, we investigate the correlation between the surface defects and the luminescent properties of the QDs.
-
Citations
Citations to this article as recorded by- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
So-Yeong Joo, Hyun-Su Park, Do-yeon Kim, Bum-Sung Kim, Chan Gi Lee, Woo-Byoung Kim
AIP Advances.2018;[Epub] CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
- [Korean]
- Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
- Il-Kyu Park
- J Korean Powder Metall Inst. 2016;23(4):270-275. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.270
- 883 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
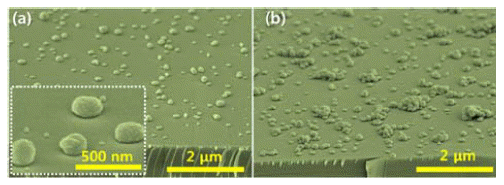
PDF We report on an all-solution-processed hydrothermal method to control the morphology of ZnO nanostructures on Si substrates from three-dimensional hemispherical structures to two-dimensional thin film layers, by controlling the seed layer and the molar contents of surfactants during their primary growth. The size and the density of the seed layer, which is composed of ZnO nanodots, change with variation in the solute concentration. The ZnO nanodots act as heterogeneous nucleation sites for the main ZnO nanostructures. When the seed layer concentration is increased, the ZnO nanostructures change from a hemispherical shape to a thin film structure, formed by densely packed ZnO hemispheres. In addition, the morphology of the ZnO layer is systematically controlled by using trisodium citrate, which acts as a surfactant to enhance the lateral growth of ZnO crystals rather than a preferential one-dimensional growth along the c-direction. X-ray diffraction and energy dispersive X-ray spectroscopy results reveal that the ZnO structure is wurtzite and did not incorporate any impurities from the surfactants used in this study.
-
Citations
Citations to this article as recorded by- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
Leeseung Kang, HyeLan An, Ji Young Park, Myung Hwan Hong, Sahn Nahm, Chan Gi Lee
Applied Surface Science.2019; 475: 969. CrossRef
- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
- [Korean]
- Influence of Sintering Temperature on Magnetic Properties of Ni-Zn-Cu Ferrites Used for Mangetic Shielding in NFC
- Yo-Han Ryu, Sung-Soo Kim
- J Korean Powder Metall Inst. 2016;23(2):132-135. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.132
- 513 View
- 2 Download
-
 Abstract
Abstract
 PDF
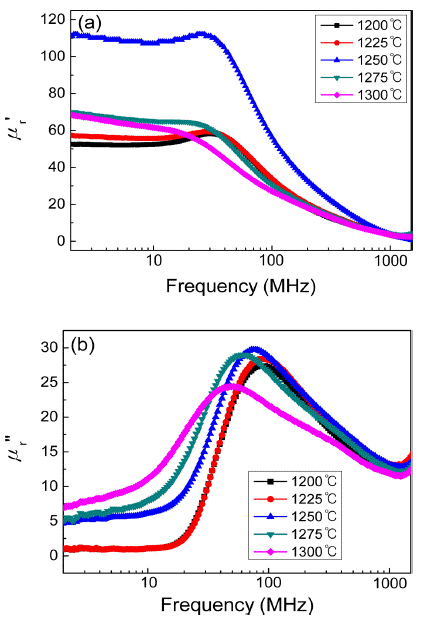
PDF This study investigates the influence of sintering temperature on the magnetic properties and frequency dispersion of the complex permeability of Ni–Zn–Cu ferrites used for magnetic shielding in near-field communication (NFC) systems. Sintered specimens of (Ni0.7Zn0.3)0.96Cu0.04Fe2O4 are prepared by conventional ceramic processing. The complex permeability is measured by an RF impedance analyzer in the range of 1 MHz to 1.8 GHz. The real and imaginary parts of the complex permeability depend sensitively on the sintering temperature, which is closely related to the microstructure, including grain size and pore distribution. In particular, internal pores within grains produced by rapid grain growth decrease the permeability and increase the magnetic loss at the operating frequency of NFC (13.56 MHz). At the optimized sintering temperature (1225-1250°C), the highest permeability and lowest magnetic loss can be obtained.
- [Korean]
- Fabrication of ZnO Nanorod/polystyrene Nanosphere Hybrid Nanostructures by Hydrothermal Method for Energy Generation Applications
- Seong-Ho Baek, Il-Kyu Park
- J Korean Powder Metall Inst. 2015;22(6):391-395. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.391

- 968 View
- 3 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF We report on the successful fabrication of ZnO nanorod (NR)/polystyrene (PS) nanosphere hybrid nanostructure by combining drop coating and hydrothermal methods. Especially, by adopting an atomic layer deposition method for seed layer formation, very uniform ZnO NR structure is grown on the complicated PS surfaces. By using zinc nitrate hexahydrate [Zn(NO3)2 ·6H2O] and hexamine [(CH2)6N4] as sources for Zn and O in hydrothermal process, hexagonal shaped single crystal ZnO NRs are synthesized without dissolution of PS in hydrothermal solution. X-ray diffraction results show that the ZnO NRs are grown along c-axis with single crystalline structure and there is no trace of impurities or unintentionally formed intermetallic compounds. Photoluminescence spectrum measured at room temperature for the ZnO NRs on flat Si and PS show typical two emission bands, which are corresponding to the band-edge and deep level emissions in ZnO crystal. Based on these structural and optical investigations, we confirm that the ZnO NRs can be grown well even on the complicated PS surface morphology to form the chestnut-shaped hybrid nanostructures for the energy generation and storage applications.
-
Citations
Citations to this article as recorded by- Synthesis of Planar-Type ZnO Powder in Non-Nano Scale Dimension and Its Application in Ultraviolet Protection Cosmetics
Jung-Hwan Lee, Gun-Sub Lee, Eung-Nam Park, Dong-Hyeon Jo, So-Won Kim, Hee-Chul Lee
Materials.2023; 16(5): 2099. CrossRef - Rapid consolidation of nanostuctured WC-FeAl3 by pulsed current activated heating and its mechanical properties
In-Jin Shon, Seok-Jae Lee
International Journal of Refractory Metals and Hard Materials.2017; 65: 69. CrossRef - Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
Young-Tae Kwon, Sung-Oong Kang, Ji-Ae Cheon, Yoseb Song, Jong-Jin Lee, Yong-Ho Choa
Applied Surface Science.2017; 415: 2. CrossRef - Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 270. CrossRef
- Synthesis of Planar-Type ZnO Powder in Non-Nano Scale Dimension and Its Application in Ultraviolet Protection Cosmetics
- [Korean]
- Fabrication of ZnO Nanorod based Robust Nanogenerator Metal Substrate
- Seong-Ho Baek, Il-Kyu Park
- J Korean Powder Metall Inst. 2015;22(5):331-336. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.331

- 1,252 View
- 6 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF We report on the succesful fabrication of ZnO nanorod (NR)-based robust piezoelectric nanogenerators (PNGs) by using Cu foil substrate. The ZnO NRs are successfully grown on the Cu foil substrate by using all solution based method, a two step hydrothermal synthesis. The ZnO NRs are grown along c-axis well with an average diameter of 75~80 nm and length of 1~1.5 μm. The ZnO NRs showed abnormal photoluminescence specrta which is attributed from surface plasmon resonance assistant enhancement at specific wavelength. The PNGs on the SUS substrates show typical piezoelectric output performance which showing a frequency dependent voltage enhancement and polarity dependent charging and discharging characteristics. The output voltage range is 0.79~2.28 V with variation of input strain frequency of 1.8~3.9 Hz. The PNG on Cu foil shows reliable output performance even at the operation over 200 times without showing degradation of output voltage. The current output from the PNG is 0.7 μA/cm2 which is a typical output range from the ZnO NR-based PNGs. These performance enhancement is attributed from the high flexibility, high electrical conductivity and excellent heat dissipation properties of the Cu foil as a substrate.
-
Citations
Citations to this article as recorded by- Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
Young-Tae Kwon, Sung-Oong Kang, Ji-Ae Cheon, Yoseb Song, Jong-Jin Lee, Yong-Ho Choa
Applied Surface Science.2017; 415: 2. CrossRef - Fabrication of Porous Polytetrafluoroethylene thin Film from Powder Dispersion-solution for Energy Nanogenerator Applications
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 102. CrossRef - Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 270. CrossRef - Fabrication of ZnO Nanorod/polystyrene Nanosphere Hybrid Nanostructures by Hydrothermal Method for Energy Generation Applications
Seong-Ho Baek, Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2015; 22(6): 391. CrossRef
- Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
- [Korean]
- Investigation on the Sintering Behavior and Mechanical Properties of Al-Zn-Mg Alloy Powders Mixed with Al-Si-SiC Composite Powders
- Gwang-Joo Jang, Kyung Tae Kim, Sangsun Yang, Yong-Jin Kim, Yong-Ho Park
- J Korean Powder Metall Inst. 2014;21(6):460-466. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.460
- 855 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
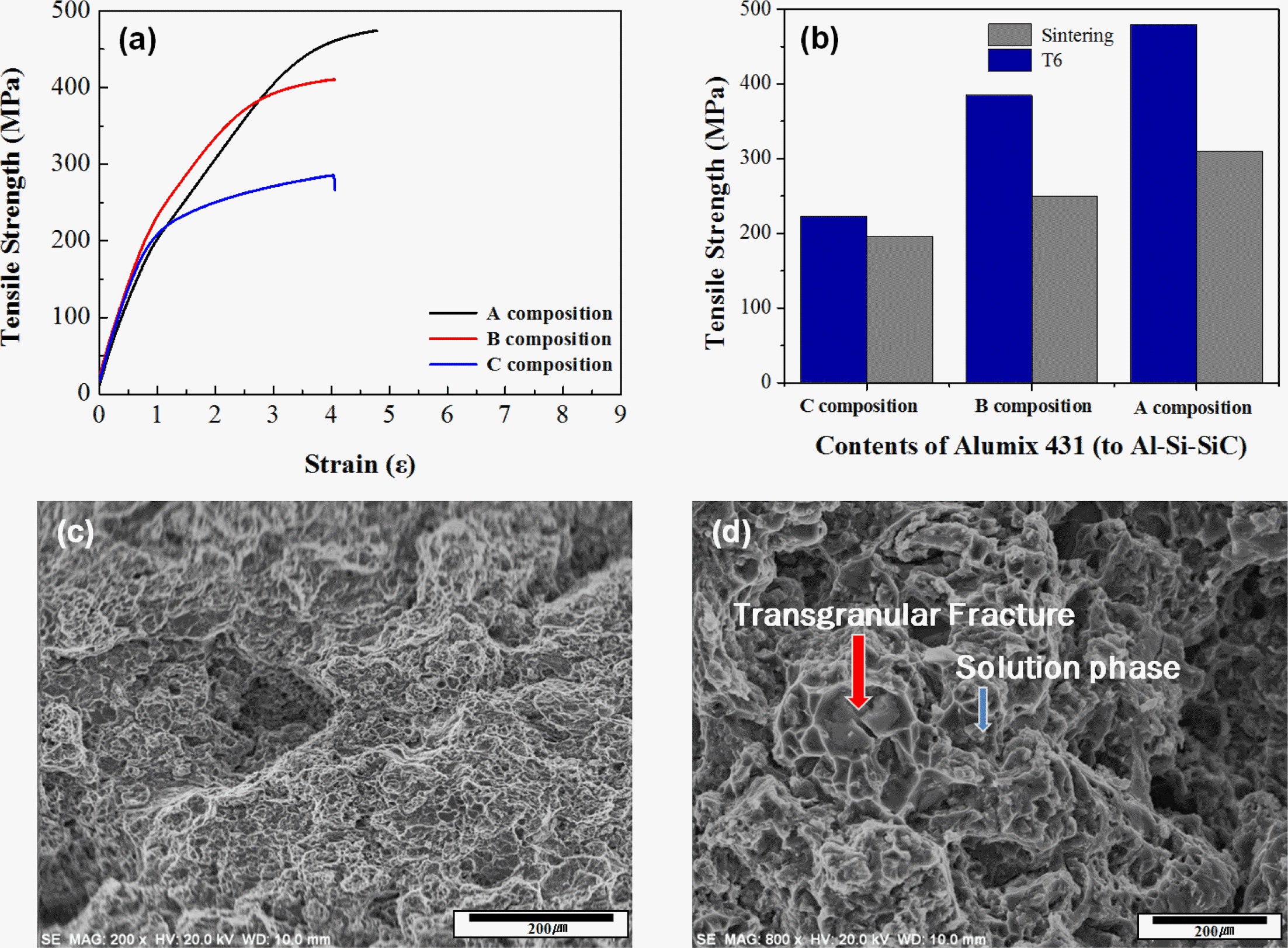
PDF Al-Si-SiC composite powders with intra-granular SiC particles were prepared by a gas atomization process. The composite powders were mixed with Al-Zn-Mg alloy powders as a function of weight percent. Those mixture powders were compacted with the pressure of 700 MPa and then sintered at the temperature of 565-585°C. T6 heat treatment was conducted to increase their mechanical properties by solid-solution precipitates. Each relative density according to the optimized sintering temperature of those powders were determined as 96% at 580°C for Al-Zn-Mg powders (composition A), 97.9% at 575°C for Al-Zn-Mg powders with 5 wt.% of Al-Si-SiC powders (composition B), and 98.2% at 570°C for Al-Zn-Mg powders with 10 wt.% of Al-Si-SiC powders (composition C), respectively. Each hardness, tensile strength, and wear resistance test of those sintered samples was conducted. As the content of Al-Si-SiC powders increased, both hardness and tensile strength were decreased. However, wear resistance was increased by the increase of Al-Si-SiC powders. From these results, it was confirmed that Al-Si-SiC/Al-Zn-Mg composite could be highly densified by the sintering process, and thus the composite could have high wear resistance and tensile strength when the content of Al-Si-SiC composite powders were optimized.
-
Citations
Citations to this article as recorded by- Effect of Tin Addition on the Melting Temperatures and Mechanical Properties of Al-Si-Cu Brazing Filler Metals
Min Sang Kim, Chun Woong Park, Jong Min Byun, Young Do Kim
Korean Journal of Materials Research.2016; 26(7): 376. CrossRef
- Effect of Tin Addition on the Melting Temperatures and Mechanical Properties of Al-Si-Cu Brazing Filler Metals
- [Korean]
- High-frequency Magnetic Properties of Ni-Zn-Co Ferrites Used for Mangetic Shielding in NFC
- Yo-Han Ryu, Sung-Soo Kim
- J Korean Powder Metall Inst. 2014;21(6):429-433. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.429
- 646 View
- 1 Download
-
 Abstract
Abstract
 PDF
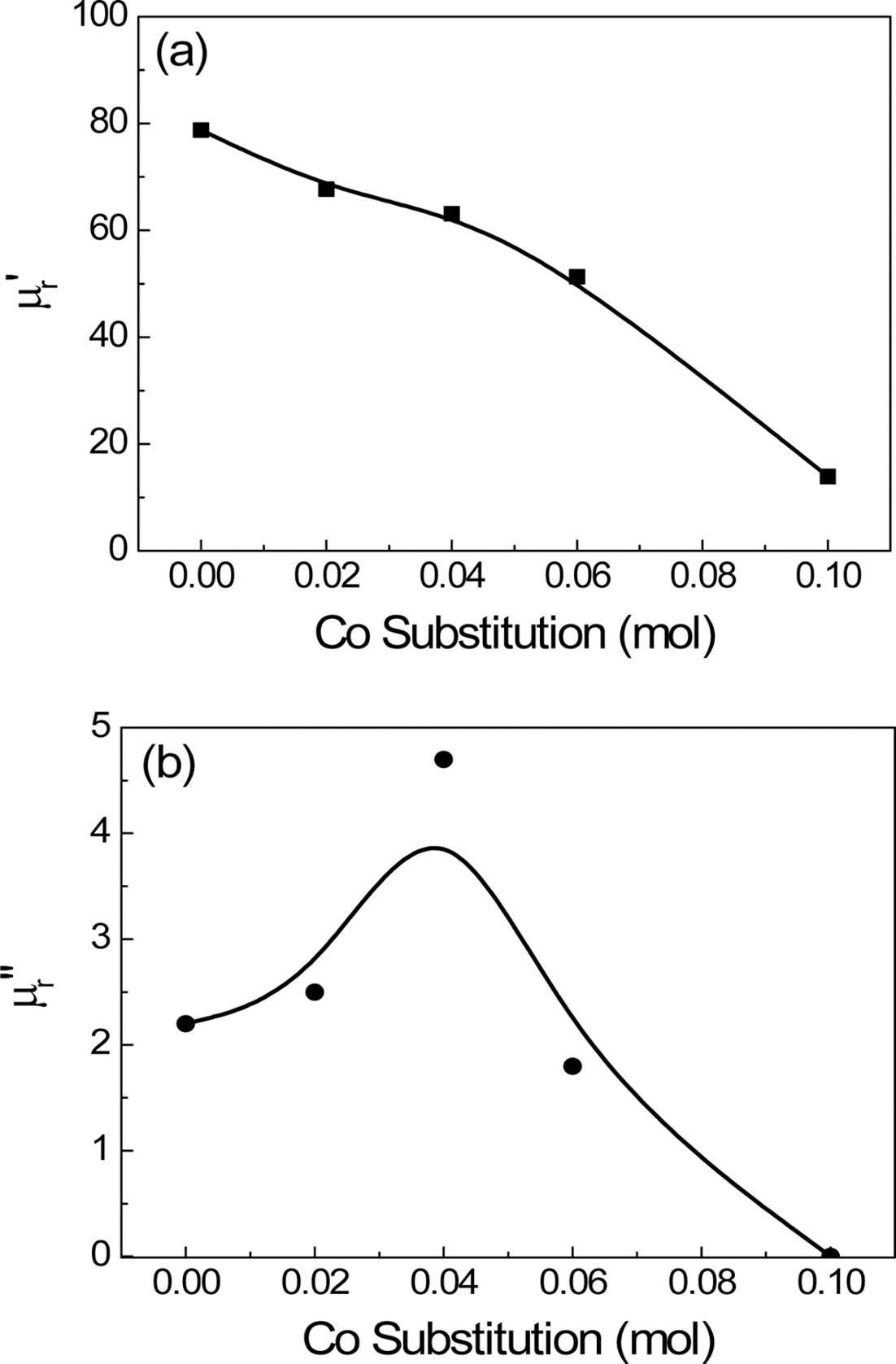
PDF This study investigated the magnetic properties and frequency dispersion of complex permeability of Ni-Zn-Co ferrites used for magnetic shielding in near field communication (NFC) system. The sintered specimens of (Ni0.7Zn0.3)1-xCoxFe2O4 composition were prepared by the conventional ceramic processing. The coercive force and saturation magnetization were measured by vibrating sample magnetometer. The complex permeability was measured by RF impedance analyzer in the range of 1 MHz~1.8 GHz. The coercive force increased and saturation magnetization decreased with increasing the Co substitution. The real and imaginary parts of complex permeability decreased and the resonance frequency increased with Co substitution, which was attributed to the increase in crystal anisotropy field and reduction in saturation magnetization. The effect of Co substitution could be found in reducing the magnetic loss to nearly zero at the operating frequency of NFC (13.56 MHz).
TOP
 KPMI
KPMI


 First
First Prev
Prev


