Search
- Page Path
- HOME > Search
- [Korean]
- Epoxy-Based Siloxane/Silica Composites for Electronic Packaging by Composition and Molecular Structure of Siloxane, and Analysis of Changes in Properties
- Junho Jang, Dong Jun Kang, Hyeon-Gyun Im
- J Powder Mater. 2023;30(4):346-355. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.346
- 1,145 View
- 14 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
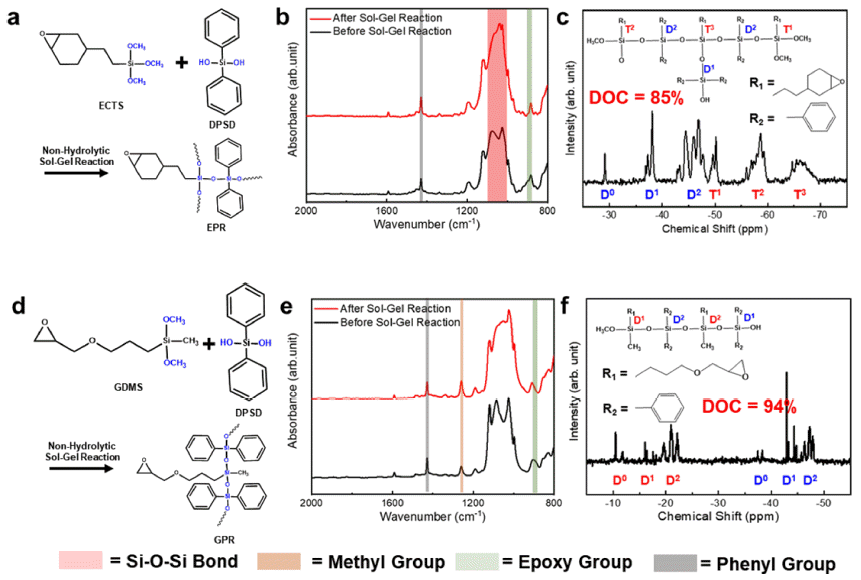
PDF Epoxy-based composites find extensive application in electronic packaging due to their excellent processability and insulation properties. However, conventional epoxy-based polymers exhibit limitations in terms of thermal properties and insulation performance. In this study, we develop epoxy-based siloxane/silica composites that enhance the thermal, mechanical, and insulating properties of epoxy resins. This is achieved by employing a sol–gelsynthesized siloxane hybrid and spherical fused silica particles. Herein, we fabricate two types of epoxy-based siloxane/ silica composites with different siloxane molecular structures (branched and linear siloxane networks) and investigate the changes in their properties for different compositions (with or without silica particles) and siloxane structures. The presence of a branched siloxane structure results in hardness and low insulating properties, while a linear siloxane structure yields softness and highly insulating properties. Both types of epoxy-based siloxane/silica composites exhibit high thermal stability and low thermal expansion. These properties are considerably improved by incorporating silica particles. We expect that our developed epoxy-based composites to hold significant potential as advanced electronic packaging materials, offering high-performance and robustness.
-
Citations
Citations to this article as recorded by- Toughening epoxy matrices via elastomeric blending for enhanced compatibility with auxetic reinforcements in composites
Usama Khalid, Shiza Shahid, Muhammad Bilal Qadir, Mumtaz Ali, Zubair Khaliq, Aamir Naseem Satti, Zaheer Anwar Randhawa, Danish Umer
Mechanics Based Design of Structures and Machines.2025; : 1. CrossRef - Enhanced Epoxy Composites Reinforced by 3D-Aligned Aluminum Borate Nanowhiskers
Hyunseung Song, Kiho Song, Haejin Hwang, Changui Ahn
Materials.2024; 17(19): 4727. CrossRef
- Toughening epoxy matrices via elastomeric blending for enhanced compatibility with auxetic reinforcements in composites
- [Korean]
- A Study of Various SiO2 Coating Control on White TiO2 Pigment for Cosmetic Applications
- Minsol Park, Wooyoung Shim, YooJin Kim
- J Powder Mater. 2022;29(3):207-212. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.207
- 1,088 View
- 24 Download
-
 Abstract
Abstract
 PDF
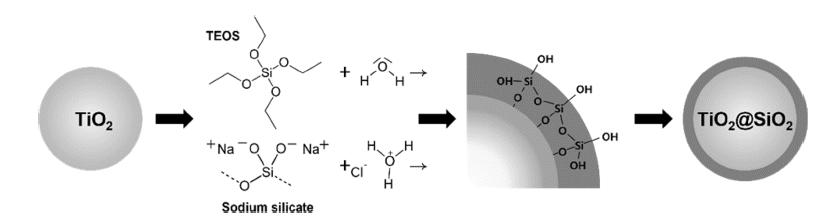
PDF Nanosized rutile titanium dioxide (TiO2) is used in inorganic pigments and cosmetics because of its high whiteness and duality. The high quality of the white pigments depends on their surface coating technique via the solgel process. SiO2 coatings are required to improve the dispersibility, UV-blocking, and whiteness of TiO2. Tetraethyl orthosilicate (TEOS) is an important coating precursor owing to its ability to control various thicknesses and densities. In addition, we use Na2SiO3 (sodium silicate) as a precursor because of its low cost. Compared to TEOS, which controls the pH using a basic catalyst, Na2SiO3 controls the pH using an acid catalyst, giving a uniform coating. The coating thickness of TiO2 is controlled using a surface modifier, cetrimonium bromide, which is used in various applications. The shape and thickness of the nanosized coating layer on TiO2 are analyzed using transmission electron microscopy, and the SiO2 nanoparticle behavior in terms of the before-and-after size distribution is measured using a particle size analyzer. The color measurements of the SiO2 pigment are performed using UV-visible spectroscopy.
- [English]
- A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
- Jei-Pil Wang, Gyu-Cheol Kim, Min-Seok Go
- J Korean Powder Metall Inst. 2020;27(4):287-292. Published online August 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.4.287
- 1,814 View
- 23 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
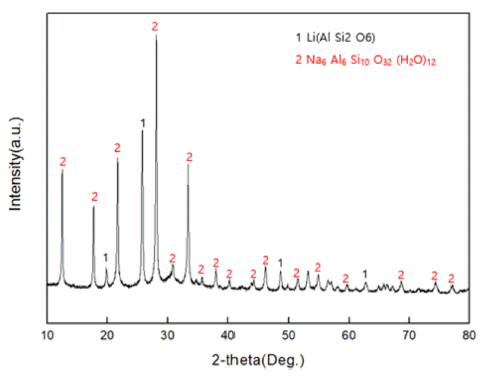
PDF The subject of this study is a zeolite generated as a by-product of recycling LAS (lithium-aluminum-silicate) resources, a kind of glass and ceramic produced by induction. The zeolite by-product is modified into Mg-zeolite using Mg as a cation to absorb Pb, a heavy metal generated from water pollution caused by recent industrial wastewater. An ion-exchange method is used to carry out the modification process, from zeolite byproduct to Mg-zeolite, and simultaneously absorb the Pb in the heavy-metal solution (99.032 mg/L). It is found that the sodium zeolite in the raw material residue can be modified to magnesium zeolite by reacting it with a mixture solution at 1 M concentration for 24 h. As a result, it is found that the residual Pb (0.130 mg/L) in the heavy metal solution is shown to be absorbed by 99.86%, with successful formation of a Mg-modified zeolite.
-
Citations
Citations to this article as recorded by- Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
Rohmat Ismail, Manasye Erlangga, Ari Himawan, Givana Indah Nurul Afiah
Jurnal Pengelolaan Laboratorium Pendidikan.2025; 7(2): 81. CrossRef - Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics
Kinjal J. Shah, Jiacheng Yu, Ting Zhang, Zhaoyang You
Water.2023; 15(6): 1171. CrossRef
- Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
- [Korean]
- Synthesis of C3S, C2S, C3A Powders using Ultra-fine Calcium Oxide Powder Synthesized from Eggshell and Effect of C3A Content on Hardened Mixed Aggregates
- Heon Kong, Ki-Beom Kwon, Sang-Jin Park, Whyo-Sub Noh, Sang-Jin Lee
- J Korean Powder Metall Inst. 2019;26(6):493-501. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.493
- 1,024 View
- 18 Download
-
 Abstract
Abstract
 PDF
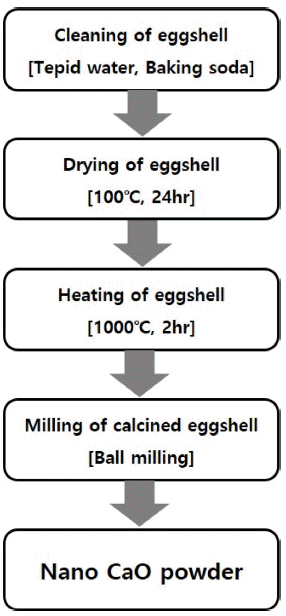
PDF In this work, ultra-fine calcium oxide (CaO) powder derived from eggshells is used as the starting material to synthesize mineral trioxide aggregate (MTA). The prepared CaO powder is confirmed to have an average particle size of 500 nm. MTAs are synthesized with three types of fine CaO-based powders, namely, tricalcium silicate (C3S), dicalcium silicate (C2S), and tricalcium aluminate (C3A). The synthesis behavior of C3S, C2S and C3A with ultra-fine CaO powder and the effects of C3A content and curing time on the properties of MTA are investigated. The characteristics of the synthesized MTA powders are examined by X-ray diffraction (XRD), field emission-scanning electron microscope (FE-SEM), and a universal testing machine (UTM). The microstructure and compressive strength characteristics of the synthesized MTA powders are strongly dependent on the C3A wt.% and curing time. Furthermore, MTA with 5 wt.% C3A is found to increase the compressive strength and shorten the curing time.
- [Korean]
- Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
- Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2018;25(5):420-425. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.420

- 923 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Core-shell structured nanoparticles are garnering attention because these nanoparticles are expected to have a wide range of applications. The objective of the present study is to improve the coating efficiency of gold shell formed on the surface of silica nanoparticles for SiO2@Au core-shell structure. For the efficient coating of gold shell, we attempt an in-situ synthesis method such that the nuclei of the gold nanoparticles are generated and grown on the surface of silica nanoparticles. This method can effectively form a gold shell as compared to the conventional method of attaching gold nanoparticles to silica particles. It is considered possible to form a dense gold shell because the problems caused by electrostatic repulsion between the gold nanoparticles in the conventional method are eliminated.
- [Korean]
- Study of Color Evolution by Silica Coating and Etching based Morphological Control of α-FeOOH
- NaRi Lee, Ri Yu, YooJin Kim
- J Korean Powder Metall Inst. 2018;25(5):379-383. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.379
- 1,178 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
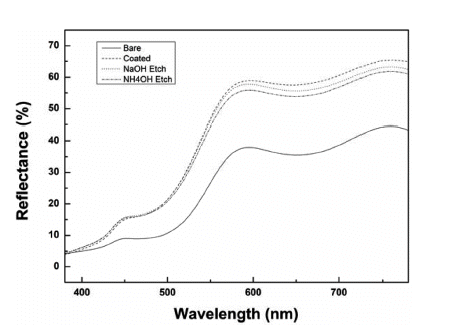
PDF Silica is used in shell materials to minimize oxidation and aggregation of nanoparticles. Particularly, porous silica has gained attention because of its performance in adsorption, catalysis, and medical applications. In this study, to investigate the effect of the density of the silica coating layer on the color of the pigment, we arbitrarily change the structure of a silica layer using an etchant. We use NaOH or NH4OH to etch the silica coating layer. First, we synthesize α-FeOOH for a length of 400 nm and coat it with TEOS to fabricate particles with a 50 nm coating layer. The coating thickness is then adjusted to 30–40 nm by etching the silica layer for 5 h. Four different shapes of α-FeOOH with different colors are measured using UV–vis light. From the color changes of the four different shapes of α-FeOOH features during coating or etching, the
L * value is observed to increase and brighten the overall color, and theb * value increases to impart a clear yellow color to the pigment. The brightest yellow color was that coated with silica; if the sample is etched with NaOH or NH4OH, theb * value can be controlled to study the yellow colors.-
Citations
Citations to this article as recorded by- Trend of Ceramic Nano Pigments
Ri Yu, YooJin Kim
Ceramist.2019; 22(3): 256. CrossRef
- Trend of Ceramic Nano Pigments
- [Korean]
- Synthesis and Characterization of Core-Shell Silica-Phosphor Nanoparticles
via Sol-Gel Process - Weon Ho Shin, Seyun Kim, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2018;25(1):12-18. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.12
- 1,001 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
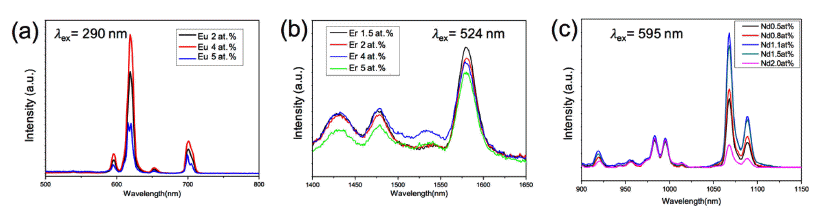
PDF Cost-effective functional phosphor nanoparticles are prepared by introducing low-cost SiO2 spheres to rareearth phosphor (YVO4:Eu3+, YVO4:Er3+, and YVO4:Nd3+) shells using a sol-gel synthetic method. These functional nanoparticles are characterized by X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, and general photoluminescence spectra. The SiO2 sphere occupying the interior of the conventional phosphor is advantageous in significantly reducing the cost of expensive rare-earth phosphor nanoparticles. The sol-gel process facilitates the core–shell structure formation; the rare-earth shell phosphor has strong interactions with chelating agents on the surfaces of SiO2 nanoparticles and thus forms layers of several nanometers in thickness. The photoluminescence wavelength is simply tuned by replacing the active materials of Eu3+, Er3+, and Nd3+. Moreover, the photoluminescent properties of the core–shell nanoparticles can be optimized by manipulating the specific contents of active materials in the phosphors. Our simple approach substitutes low-cost SiO2 for expensive rare-earth-based phosphor materials to realize cost-effective phosphor nanoparticles for various applications.
-
Citations
Citations to this article as recorded by- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
Woo Hyeong Sim, Seyun Kim, Weon Ho Shin, Hyung Mo Jeong
Korean Journal of Metals and Materials.2020; 58(2): 137. CrossRef
- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
- [Korean]
- Effect of Deposition Parameter and Mixing Process of Raw Materials on the Phase and Structure of Ytterbium Silicate Environmental Barrier Coatings by Suspension Plasma Spray Method
- Ho-lim Ryu, Seon-A Choi, Sung-Min Lee, Yoon-Soo Han, Kyun Choi, Sahn Nahm, Yoon-Suk Oh
- J Korean Powder Metall Inst. 2017;24(6):437-443. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.437

- 822 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF SiC-based composite materials with light weight, high durability, and high-temperature stability have been actively studied for use in aerospace and defense applications. Moreover, environmental barrier coating (EBC) technologies using oxide-based ceramic materials have been studied to prevent chemical deterioration at a high temperature of 1300°C or higher. In this study, an ytterbium silicate material, which has recently been actively studied as an environmental barrier coating because of its high-temperature chemical stability, is fabricated on a sintered SiC substrate. Yb2O3 and SiO2 are used as the raw starting materials to form ytterbium disilicate (Yb2Si2O7). Suspension plasma spraying is applied as the coating method. The effect of the mixing method on the particle size and distribution, which affect the coating formation behavior, is investigated using a scanning electron microscope (SEM), an energy dispersive spectrometer (EDS), and X-ray diffraction (XRD) analysis. It is found that the originally designed compounds are not effectively formed because of the refinement and vaporization of the raw material particles, i.e., SiO2, and the formation of a porous coating structure. By changing the coating parameters such as the deposition distance, it is found that a denser coating structure can be formed at a closer deposition distance.
-
Citations
Citations to this article as recorded by- Fabrication, Microstructure and Adhesive Properties of BCuP-5 Filler Metal/Ag Plate Composite by using Plasma Spray Process
Seong-June Youn, Young-Kyun Kim, Jae-Sung Park, Joo-Hyun Park, Kee-Ahn Lee
Journal of Korean Powder Metallurgy Institute.2020; 27(4): 333. CrossRef
- Fabrication, Microstructure and Adhesive Properties of BCuP-5 Filler Metal/Ag Plate Composite by using Plasma Spray Process
- [English]
- Lithium-silicate coating on Lithium Nickel Manganese Oxide (LiNi0.7Mn0.3O2) with a Layered Structure
- Dong-jin Kim, Da-ye Yoon, Woo-byoung Kim, Jae-won Lee
- J Korean Powder Metall Inst. 2017;24(2):87-95. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.87
- 1,299 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
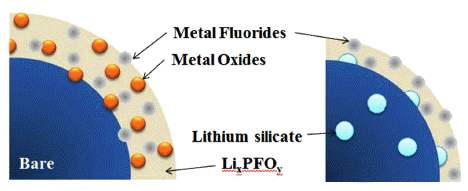
PDF Lithium silicate, a lithium-ion conducting ceramic, is coated on a layer-structured lithium nickel manganese oxide (LiNi0.7Mn0.3O2). Residual lithium compounds (Li2CO3 and LiOH) on the surface of the cathode material and SiO2 derived from tetraethylorthosilicate are used as lithium and silicon sources, respectively. Powder X-ray diffraction and scanning electron microscopy with energy-dispersive spectroscopy analyses show that lithium silicate is coated uniformly on the cathode particles. Charge and discharge tests of the samples show that the coating can enhance the rate capability and cycle life performance. The improvements are attributed to the reduced interfacial resistance originating from suppression of solid-electrolyte interface (SEI) formation and dissolution of Ni and Mn due to the coating. An X-ray photoelectron spectroscopy study of the cycled electrodes shows that nickel oxide and manganese oxide particles are formed on the surface of the electrode and that greater decomposition of the electrolyte occurs for the bare sample, which confirms the assumption that SEI formation and Ni and Mn dissolution can be reduced using the coating process.
-
Citations
Citations to this article as recorded by- Artificial cathode-electrolyte interphases on nickel-rich cathode materials modified by silyl functional group
Hye Ji Song, Seol Heui Jang, Juhyeon Ahn, Si Hyoung Oh, Taeeun Yim
Journal of Power Sources.2019; 416: 1. CrossRef
- Artificial cathode-electrolyte interphases on nickel-rich cathode materials modified by silyl functional group
- [Korean]
- Effect of Reaction Parameters on Silica Nanoparticles Synthesized by Sol-gel Method
- Young-Hyun Lim, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2016;23(6):442-446. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.442
- 3,027 View
- 47 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
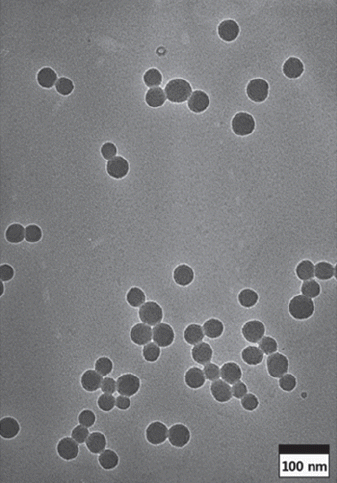
PDF The sol-gel method is the simplest method for synthesizing monodispersed silica particles. The purpose of this study is to synthesize uniform, monodisperse spherical silica nanoparticles using tetraethylorthosilicate (TEOS) as the silica precursor, ethanol, and deionized water in the presence of ammonia as a catalyst. The reaction time and temperature and the concentration of the reactants are controlled to investigate the effect of the reaction parameters on the size of the synthesized particles. The size and morphology of the obtained silica particles are investigated using transmission electron microscopy and particle size analysis. The results show that monodispersed silica particles over a size range of 54-504 nm are successfully synthesized by the sol-gel method without using any additional process. The nanosized silica particles can be synthesized at higher TEOS/H2O ratios, lower ammonia concentrations, and especially, higher reaction temperatures.
-
Citations
Citations to this article as recorded by- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
Mustapha Sulaiman, Naseer Inuwa Durumin Iya, Mamudu Aliyu
FUDMA JOURNAL OF SCIENCES.2024; 8(3): 222. CrossRef - Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 420. CrossRef
- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
- [Korean]
- Synthesis of KIT-1 Mesoporous Silicates Showing Two Different Macrosporous Strucrtues; Inverse-opal or Hollow Structures
- Youn-Kyoung Baek, Jung-Goo Lee, Young Kuk Kim
- J Korean Powder Metall Inst. 2016;23(3):189-194. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.189
- 596 View
- 1 Download
-
 Abstract
Abstract
 PDF
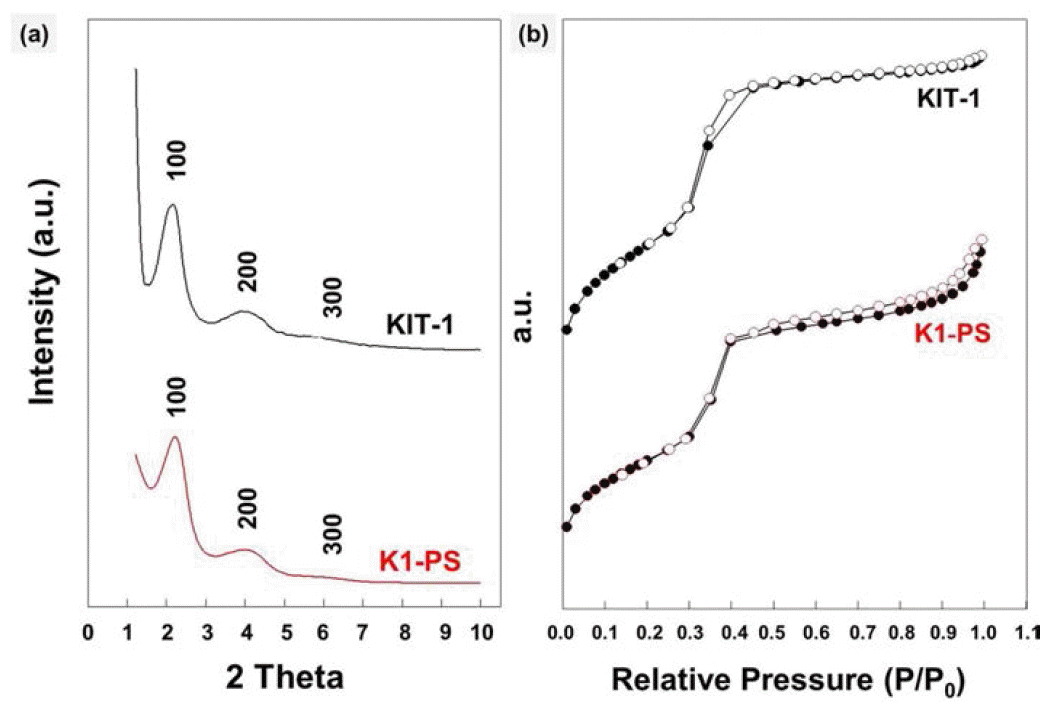
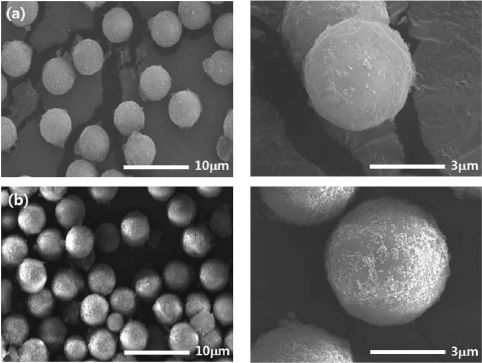
PDF We report a facile method for preparing KIT-1 mesoporous silicates with two different macroporous structures by dual templating. As a template for macropores, polystyrene (PS) beads are assembled into uniform three dimensional arrays by ice templating, i.e., by growing ice crystals during the freezing process of the particle suspension. Then, the polymeric templates are directly introduced into the precursor-gel solution with cationic surfactants for templating the mesopores, which is followed by hydrothermal crystallization and calcination. Later, by burning out the PS beads and the surfactants, KIT-1 mesoporous silicates with macropores are produced in a powder form. The macroporous structures of the silicates can be controlled by changing the amount of EDTANa4 salt under the same templating conditions using the PS beads and inverse-opal or hollow structures can be obtained. This strategy to prepare mesoporous powders with controllable macrostructures is potentially useful for various applications especially those dealing with bulky molecules such as, catalysis, separation, drug carriers and environmental adsorbents.
- [Korean]
- Dispersion Control and Characterization of the SiO2/PMMA Particles Using Surface Charge
- Yubin Kang, Soojung Son, Kun-Jae Lee
- J Korean Powder Metall Inst. 2015;22(6):403-407. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.403
- 985 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Poly-methylmetacrylate (PMMA) is mainly applied in the plastic manufacturing industry, but PMMA is weak and gradually got discolor. The strength of PMMA can be improved through organic-inorganic hybrid nano composites with inorganic nano particles such as, SiO2 or ZrO. However, inorganic nano particles are mostly agglomerated spontaneously. In this study, the zeta potential is controlled using different types of organic solvent with different concentrations, dispersibillity of SiO2 nano particles on the PMMA particle are analyzed. When 3 M acetic acid is used, absolute value of the zeta potential is higher, SiO2 nano particle is well attached, and dispersed on the PMMA particle surface. Results indicate that the absolute value of the zeta potential affects the stability of SiO2 dispersion.
-
Citations
Citations to this article as recorded by- A Study of Organic Impurity Removal Efficiency for Waste LCD Touch Panel Glass by Solvents Types
Yubin Kang, Jin-Ju Choi, Jae Layng Park, Chan Gi Lee
Journal of the Korean Institute of Resources Recycling.2020; 29(6): 57. CrossRef - Hard Surface-adhesive Properties of TiO2 Nanoparticles-encapsulated Microparticles Prepared by Spray Drying and Surface Coating Method
Su-Kyung Kim, Jong-Duk Kim, Seung-Jun Lee
Fibers and Polymers.2018; 19(6): 1303. CrossRef
- A Study of Organic Impurity Removal Efficiency for Waste LCD Touch Panel Glass by Solvents Types
- [Korean]
- Characterization of the Silica Coated Diatomite Based Ceramic Filter for Water Treatment
- Byung-Seo Bae, Jang-Hoon Ha, In-Hyuck Song, Yoo-Dong Hahn
- J Korean Powder Metall Inst. 2014;21(1):21-27. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.21

- 556 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this study, diatomite based materials were investigated as a support filter for silica particle coating. The silica sol for coating was synthesized by a stöber process. The diatomite support was dry-pressed at 10 MPa and sintered at 1200°C for 1 hour. The coating sol was prepared as a mixture of EtOH and silica sol. The diatomite support was coated by a dip-coating process. Silica coated diatomite filter was sintered at 1000~1200°C for 1 hour. The largest pore size was decreased with increasing concentration ratio of coating sol. The gas and water permeability of silica coated diatomite decreased with increasing of concentration ratio of the coating sol.
TOP
 KPMI
KPMI


 First
First Prev
Prev


