Search
- Page Path
- HOME > Search
- [Korean]
- Smelting and Recycling of Niobium
- Ho-Sang Sohn
- J Powder Mater. 2025;32(6):517-528. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00367
- 480 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF - Global annual production of niobium is only around 100,000 tonnes; however, it is a critical metal for modern industry and is mined in only a limited number of regions. This study reviews the current status of niobium smelting and recycling technologies. Approximately 90% of niobium is produced as ferroniobium (FeNb) for use in steel alloys, although niobium is also utilized in superalloys, superconductors, capacitors, semiconductors, and other applications. Niobium coexists with tantalum in columbite and tantalite ores. These ores are decomposed by hydrofluoric acid digestion or alkali fusion, followed by solvent extraction to separate Nb2O5 and Ta2O5. Niobium metal and FeNb are produced from Nb2O5 primarily via aluminothermic reduction, although metallic niobium can also be manufactured by thermal reduction using Mg, Ca, or C, as well as by molten salt electrolysis. Crude niobium is subsequently refined into high-purity niobium through molten salt electrolytic refining, high-temperature vacuum treatment, and electron beam melting. Because most niobium is used as an alloying element in stainless steel and high-strength low-alloy steel, recycling practices for niobium remain poorly documented.
- [Korean]
- The Recycling Process and Powderization Technology of Stellite 6 Scrap: A Thermodynamic and Heat Transfer Analysis
- YongKwan Lee, Hyun-chul Kim, Myungsuk Kim, Soong Ju Oh, Kyoungtae Park, JaeJin Sim
- J Powder Mater. 2025;32(4):330-343. Published online August 29, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00136
- 917 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - Co-Cr alloys are widely used in cutting tools and turbine components due to their high strength and resistance against wear and corrosion. However, scrap generated during hardfacing is often discarded due to impurities and oxidation, and research on its recycling remains limited. This study aimed to optimize the recycling process of Stellite 6 scrap to reduce waste and minimize costs while maintaining material quality. Melting, casting, and powdering processes were designed using HSC Chemistry, FactSage, and COMSOL Multiphysics, with optimization of key parameters such as the crucible material and temperature control. The recycled alloy and powder were analyzed using X-ray fluorescence analysis, inductively coupled plasma optical emission spectroscopy, and X-ray diffractometry, showing mechanical and chemical properties comparable to commercial Stellite 6. The Co and Cr contents were maintained, with a slight increase in Fe. These findings demonstrate the potential for producing high-quality recycled Stellite 6 materials, contributing to the sustainable utilization of metal resources in high-performance applications.
- [English]
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- Haein Shin, Kun-Jae Lee
- J Powder Mater. 2024;31(5):374-381. Published online October 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00192
- 1,885 View
- 48 Download
-
 Abstract
Abstract
 PDF
PDF - The development of the electronics industry has led to an increased demand for the manufacture of MLCC (Multilayer Ceramic Capacitors), which in turn is expected to result in a rise in MLCC waste. The MLCC contains various metals, notably barium, titanium, and nickel, whose disposal is anticipated to increase correspondingly. Recently, recycling technologies for electronic waste have garnered attention as they address waste management and raw material supply challenges. This paper investigates the recovery of barium, nickel, and titanium from the MLCC by a hydrometallurgical process. Using citric acid, which is an organic acid, the metal inside the MLCC was leached. Additionally, metal materials were recovered through precipitation and complexing processes. As a result, barium and titanium were recovered from the leachate of the waste MLCC, and 93% of the nickel-based powder was recovered. Furthermore, the optimal recovery process conditions for recycling these metal elements were investigated.
- [Korean]
- Fabrication of Nanowire by Electrospinning Process Using Nickel Oxide Particle Recovered from MLCC
- Haein Shin, Jongwon Bae, Minsu Kang, Kun-Jae Lee
- J Powder Mater. 2023;30(6):502-508. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.502
- 643 View
- 13 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF With the increasing demand for electronic products, the amount of multilayer ceramic capacitor (MLCC) waste has also increased. Recycling technology has recently gained attention because it can simultaneously address raw material supply and waste disposal issues. However, research on recovering valuable metals from MLCCs and converting the recovered metals into high-value-added materials remains insufficient. Herein, we describe an electrospinning (E-spinning) process to recover nickel from MLCCs and modulate the morphology of the recovered nickel oxide particles. The nickel oxalate powder was recovered using organic acid leaching and precipitation. Nickel oxide nanoparticles were prepared via heat treatment and ultrasonic milling. A mixture of nickel oxide particles and polyvinylpyrrolidone (PVP) was used as the E-spinning solution. A PVP/NiO nanowire composite was fabricated via Espinning, and a nickel oxide nanowire with a network structure was manufactured through calcination. The nanowire diameters and morphologies are discussed based on the nickel oxide content in the E-spinning solution.
-
Citations
Citations to this article as recorded by- Morphological Control and Surface Modification Characteristics of Nickel Oxalate Synthesized via Oxalic Acid Precipitation
Eunbi Park, Jongwon Bae, Sera Kang, Minsu Kang, Suseong Lee, Kun-Jae Lee
Journal of Powder Materials.2025; 32(5): 375. CrossRef
- Morphological Control and Surface Modification Characteristics of Nickel Oxalate Synthesized via Oxalic Acid Precipitation
- [Korean]
- Extractive Metallurgy and Recycling of Cobalt
- Ho-Sang Sohn
- J Powder Mater. 2022;29(3):252-261. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.252
- 1,055 View
- 13 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
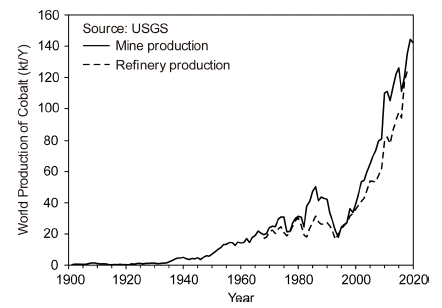
PDF Cobalt is a vital metal in the modern society because of its applications in lithium-ion batteries, super alloys, hard metals, and catalysts. Further, cobalt is a representative rare metal and is the 30th most abundant element in the Earth’s crust. This study reviews the current status of cobalt extraction and recycling processes, along with the trends in its production amount and use. Although cobalt occurs in a wide range of minerals, such as oxides and sulfides of copper and nickel ores, the amounts of cobalt in the minerals are too low to be extracted economically. The Democratic Republic of Congo (DRC) leads cobalt mining, and accounts for 68.9 % of the global cobalt reserves (142,000 tons in 2020). Cobalt is mainly extracted from copper–cobalt and nickel–cobalt concentrates and is occasionally extracted directly from the ore itself by hydro-, pyro-, and electro-metallurgical processes. These smelting methods are essential for developing new recycling processes to extract cobalt from secondary resources. Cobalt is mainly recycled from lithium-ion batteries, spent catalysts, and cobalt alloys. The recycling methods for cobalt also depend on the type of secondary cobalt resource. Major recycling methods from secondary resources are applied in pyro- and hydrometallurgical processes.
-
Citations
Citations to this article as recorded by- Reduction Behavior of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries by CO Gas
Sang-Yeop Lee, Jae-Ho Hwang, So-Yeong Lee, Ho-Sang Sohn
Korean Journal of Metals and Materials.2025; 63(10): 820. CrossRef - Recovering cobalt from cobalt oxide ore using suspension roasting and magnetic separation technique
Xinlei Wei, Yongsheng Sun, Yanjun Li, Peng Gao
Journal of Materials Research and Technology.2023; 27: 3005. CrossRef
- Reduction Behavior of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries by CO Gas
- [Korean]
- Recycling of Hardmetal Tool through Alkali Leaching Process and Fabrication Process of Nano-sized Tungsten Carbide Powder using Self-propagation High-temperature Synthesis
- Hee-Nam Kang, Dong Il Jeong, Young Il Kim, In Yeong Kim, Sang Cheol Park, Cheol Woo Nam, Seok-Jun Seo, Jin Yeong Lee, Bin Lee
- J Powder Mater. 2022;29(1):47-55. Published online February 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.1.47

- 1,307 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF Tungsten carbide is widely used in carbide tools. However, its production process generates a significant number of end-of-life products and by-products. Therefore, it is necessary to develop efficient recycling methods and investigate the remanufacturing of tungsten carbide using recycled materials. Herein, we have recovered 99.9% of the tungsten in cemented carbide hard scrap as tungsten oxide via an alkali leaching process. Subsequently, using the recovered tungsten oxide as a starting material, tungsten carbide has been produced by employing a self-propagating high-temperature synthesis (SHS) method. SHS is advantageous as it reduces the reaction time and is energy-efficient. Tungsten carbide with a carbon content of 6.18 wt % and a particle size of 116 nm has been successfully synthesized by optimizing the SHS process parameters, pulverization, and mixing. In this study, a series of processes for the highefficiency recycling and quality improvement of tungsten-based materials have been developed.
- [Korean]
- Cobalt Recovery by Oxalic Acid and Hydroxide Precipitation from Waste Cemented Carbide Scrap Cobalt Leaching Solution
- Jaesung Lee, Mingoo Kim, Seulgi Kim, Dongju Lee
- J Korean Powder Metall Inst. 2021;28(6):497-501. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.497
- 1,150 View
- 14 Download
-
 Abstract
Abstract
 PDF
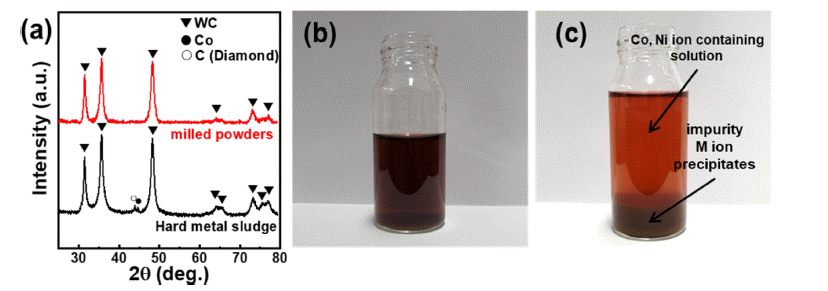
PDF Cobalt (Co) is mainly used to prepare cathode materials for lithium-ion batteries (LIBs) and binder metals for WC-Co hard metals. Developing an effective method for recovering Co from WC-Co waste sludge is of immense significance. In this study, Co is extracted from waste cemented carbide soft scrap via mechanochemical milling. The leaching ratio of Co reaches approximately 93%, and the leached solution, from which impurities except nickel are removed by pH titration, exhibits a purity of approximately 97%. The titrated aqueous Co salts are precipitated using oxalic acid and hydroxide precipitation, and the effects of the precipitating agent (oxalic acid and hydroxide) on the cobalt microstructure are investigated. It is confirmed that the type of Co compound and the crystal growth direction change according to the precipitation method, both of which affect the microstructure of the cobalt powders. This novel mechanochemical process is of significant importance for the recovery of Co from waste WC-Co hard metal. The recycled Co can be applied as a cemented carbide binder or a cathode material for lithium secondary batteries.
- [Korean]
- Fabrication of TiO2 Coated Si Nano Particle using Silicon Sawing Sludge
- Dong Hyeok Seo, Hyeon Min Yim, Ho Yoon Na, Won Jin Kim, Ryun Na Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2021;28(5):423-428. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.423

- 615 View
- 10 Download
-
 Abstract
Abstract
 PDF
PDF Here, we report the development of a new and low-cost core-shell structure for lithium-ion battery anodes using silicon waste sludge and the Ti-ion complex. X-ray diffraction (XRD) confirmed the raw waste silicon sludge powder to be pure silicon without other metal impurities and the particle size distribution is measured to be from 200 nm to 3 μm by dynamic light scattering (DLS). As a result of pulverization by a planetary mill, the size of the single crystal according to the Scherrer formula is calculated to be 12.1 nm, but the average particle size of the agglomerate is measured to be 123.6 nm. A Si/TiO2 core-shell structure is formed using simple Ti complex ions, and the ratio of TiO2 peaks increased with an increase in the amount of Ti ions. Transmission electron microscopy (TEM) observations revealed that TiO2 coating on Si nanoparticles results in a Si-TiO2 core-shell structure. This result is expected to improve the stability and cycle of lithium-ion batteries as anodes.
- [Korean]
- Current Status of Smelting and Recycling Technologies of Tungsten
- Ho-Sang Sohn
- J Korean Powder Metall Inst. 2021;28(4):342-351. Published online August 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.4.342

- 1,566 View
- 37 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Because of its unique properties, tungsten is a strategic and rare metal used in various industrial applications. However, the world's annual production of tungsten is only 84000 t. Ammonium paratungstate (APT), which is used as the main intermediate in industrial tungsten production, is usually obtained from tungsten concentrates of wolframite and scheelite by hydrometallurgical treatment. Intermediates such as tungsten trioxide, tungsten blue oxide, tungstic acid, and ammonium metatungstate can be derived from APT by thermal decomposition or chemical attack. Tungsten metal powder is produced through the hydrogen reduction of high-purity tungsten oxides, and tungsten carbide powder is produced by the reaction of tungsten powder and carbon black powder at 1300–1700°C in a hydrogen atmosphere. Tungsten scrap can be divided into hard and soft scrap based on shape (bulk or powder). It can also be divided into new scrap generated during the production of tungsten-bearing goods and old scrap collected at the end of life. Recycling technologies for tungsten can be divided into four main groups: direct, chemical, and semi-direct recycling, and melting metallurgy. In this review, the current status of tungsten smelting and recycling technologies is discussed.
-
Citations
Citations to this article as recorded by- Synthesis and thermal properties of single-phase AMT crystals via alcoholic precipitation
Haoyu Liu, Wendi Zhang, Qiusheng Zhou, Xiaobin Li, Jie Li, Guihua Liu, Zhihong Peng, Tiangui Qi, Leiting Shen, Yilin Wang
Canadian Metallurgical Quarterly.2025; : 1. CrossRef - The Current Status and Securing Strategies of Core Mineral Tungsten Resources
Dohyun Jeong, Seongmin Kim, Hoseok Jeon
Journal of the Korean Society of Mineral and Energy Resources Engineers.2023; 60(5): 341. CrossRef - Tungsten distribution and vertical migration in soils near a typical abandoned tungsten smelter
Huihui Du, Yang Li, Dan Wan, Chuanqiang Sun, Jing Sun
Journal of Hazardous Materials.2022; 429: 128292. CrossRef
- Synthesis and thermal properties of single-phase AMT crystals via alcoholic precipitation
- [English]
- Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process
- Sangmin Park, Sun-Woo Nam, Sang-Hoon Lee, Myung-Suk Song, Taek-Soo Kim
- J Korean Powder Metall Inst. 2021;28(2):91-96. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.91

- 1,541 View
- 21 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF Rare earth magnets with excellent magnetic properties are indispensable in the electric device, wind turbine, and e-mobility industries. The demand for the development of eco-friendly recycling techniques has increased to realize sustainable green technology, and the supply of rare earth resources, which are critical for the production of permanent magnets, are limited. Liquid metal extraction (LME), which is a type of pyrometallurgical recycling, is known to selectively extract the metal forms of rare earth elements. Although several studies have been carried out on the formation of intermetallic compounds and oxides, the effect of oxide formation on the extraction efficiency in the LME process remains unknown. In this study, microstructural and phase analyses are conducted to confirm the oxidation behavior of magnets pulverized by a jaw crusher. The LME process is performed with pulverized scrap, and extraction percentages are calculated to confirm the effect of the oxide phases on the extraction of Dy during the reaction. During the LME p rocess, Nd i s completely e xtracted a fter 6 h, w hile D y remains as D y2Fe17 and Dy-oxide. Because the decomposition rate of Dy2Fe17 is faster than the reduction rate of Dy-oxide, the importance of controlling Dy-oxide on Dy extraction is confirmed.
-
Citations
Citations to this article as recorded by- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
Sangmin Park, Yoonhyung Keum, Jaeyun Jeong, Seunghun Cha, Ju-Young Cho, Hyunchul Kim, Jiseong Lee, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Journal of Alloys and Compounds.2025; 1022: 178711. CrossRef - A Review of the Current Progress in High-Temperature Recycling Strategies for Recovery of Rare-Earth Elements from Magnet Waste
Ali Zakeri, Leili Tafaghodi
Journal of Sustainable Metallurgy.2025; 11(1): 88. CrossRef - Selective growth of Nb–Fe–B intermetallic compounds for the direct separation of rare earths based on manipulating liquation
Sangmin Park, Jaeyun Jeong, Seunghun Cha, Yoonhyung Keum, Ju-Young Cho, Hyungbeen Park, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Materials Today Sustainability.2024; 28: 101042. CrossRef - Separation and recovery Nd and Dy from Mg-REEs alloy by vacuum distillation
Sangmin Park, Dae-Kyeom Kim, Jaeyun Jeong, Jae Hong Shin, Yujin Kang, Rongyu Liu, Taek-Soo Kim, Myungsuk Song
Journal of Alloys and Compounds.2023; 967: 171775. CrossRef - The Supported Boro-Additive Effect for the Selective Recovery of Dy Elements from Rare-Earth-Elements-Based Magnets
Sangmin Park, Dae-Kyeom Kim, Javid Hussain, Myungsuk Song, Taek-Soo Kim
Materials.2022; 15(9): 3032. CrossRef - Influence of Dysprosium Compounds on the Extraction Behavior of Dy from Nd-Dy-Fe-B Magnet Using Liquid Magnesium
Sun-Woo Nam, Sang-Min Park, Mohammad Zarar Rasheed, Myung-Suk Song, Do-Hyang Kim, Taek-Soo Kim
Metals.2021; 11(9): 1345. CrossRef
- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
- [English]
- Synthesis of Nanosized Nickel Particle from Spent Cathodic Material Containing Lithium
- Jei-Pil Wang
- J Korean Powder Metall Inst. 2019;26(4):340-344. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.340
- 701 View
- 4 Download
-
 Abstract
Abstract
 PDF
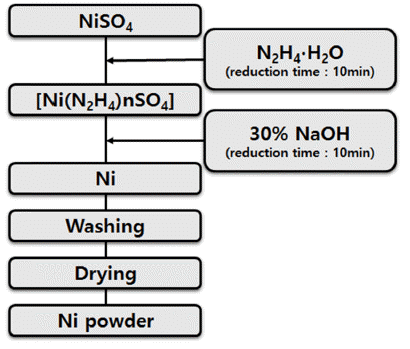
PDF Due to the rapid development of electricity, electronics, information communication, and biotechnology in recent years, studies are actively being conducted on nanopowders as it is required not only for high strengthening but also for high-function powder with electric, magnetic, and optical properties. Nonetheless, studies on nickel nanopowders are rare. In this study of the synthesis of nickel nanoparticles from LiNiO2 (LNO), which is a cathode active material, we have synthesized the nanosized nickel powder by the liquid reduction process of NiSO4 obtained through the leaching and purification of LNO. Moreover, we have studied the reduction reaction rate according to the temperature change of liquid phase reduction and the change of particle size as a function of NaOH addition amount using hydrazine monohydrate (N2H4·H2O) and NaOH.
- [Korean]
- Fabrication of WC/Co composite powder from oxide of WC/Co hardmetal scrap by carbothermal reduction process
- Gil-Geun Lee, Young Soo Lim
- J Korean Powder Metall Inst. 2018;25(3):240-245. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.240
- 665 View
- 4 Download
-
 Abstract
Abstract
 PDF
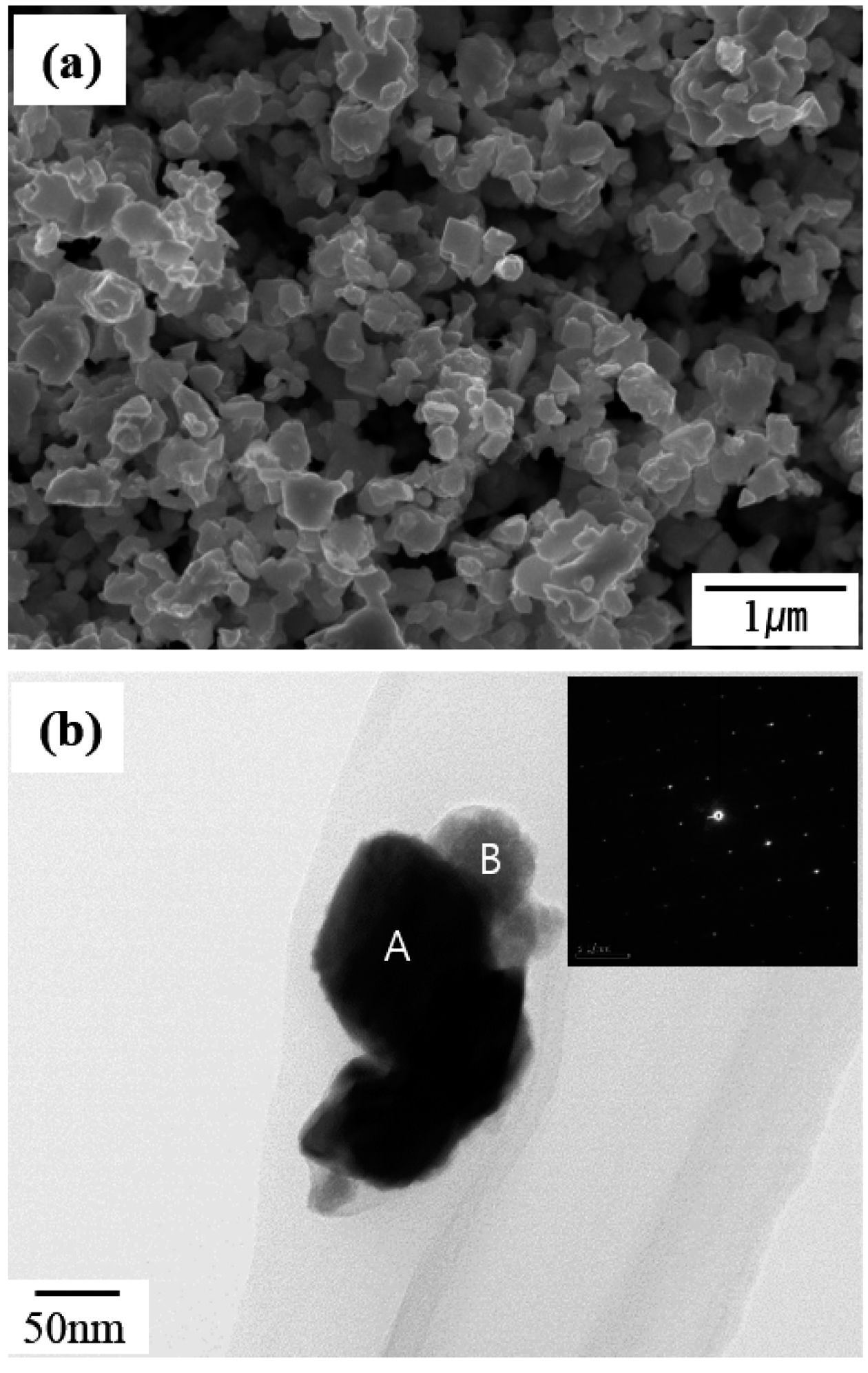
PDF This study focuses on the fabrication of a WC/Co composite powder from the oxide of WC/Co hardmetal scrap using solid carbon in a hydrogen gas atmosphere for the recycling of WC/Co hardmetal. Mixed powders are manufactured by mechanically milling the oxide powder of WC-13 wt% Co hardmetal scrap and carbon black with varying powder/ball weight ratios. The oxide powder of WC-13 wt% Co hardmetal scrap consists of WO3 and CoWO4. The mixed powder mechanically milled at a lower powder/ball weight ratio (high mechanical milling energy) has a more rapid carbothermal reduction reaction in the formation of WC and Co phases compared with that mechanically milled at a higher powder/ball weight ratio (lower mechanical milling energy). The WC/Co composite powder is fabricated at 900°C for 6 h from the oxide of WC/Co hardmetal scrap using solid carbon in a hydrogen gas atmosphere. The fabricated WC/Co composite powder has a particle size of approximately 0.25-0.5 μm.
- [Korean]
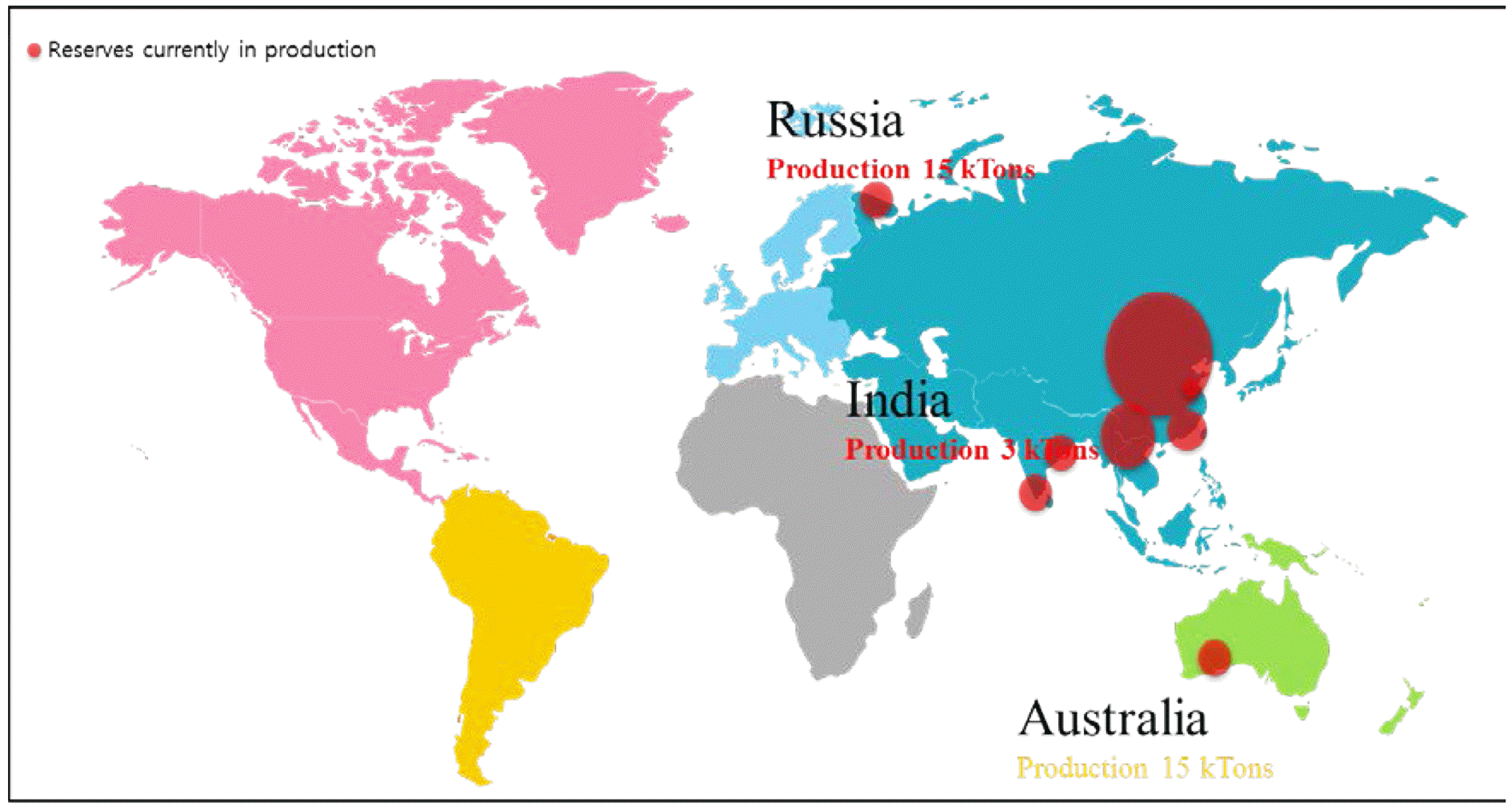
- Trends and Implications of International Standardization for Rare Earths
- Sardar Farhat Abbas, Sang-Hyun lee, Bin Lee, Bum-Sung Kim, Taek-Soo Kim
- J Korean Powder Metall Inst. 2018;25(2):165-169. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.165
- 922 View
- 6 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Rare earth elements (REEs) are considered to be vital to modern industry due to their important roles in applications such as permanent magnets, automobile production, displays, and many more. The imbalance between demand and supply of REEs can be solved by recycling processes. Regarding the needs of industry and society, the International Organization for Standardization, Technical Committee 298 (ISO/TC298) Rare Earths has been recently launched for developing international standards on rare earth elements. In accordance with the suggestion of its constituents, it is tentatively working to develop the appropriate standards under five working groups (WG) on terms and definitions (WG1), element recycling (WG2), environmental stewardship (WG3), packaging, labelling, marking, transport, and storage (WG4), and testing analysis (WG5). The scope and structure of ISO/TC298 on the topic of rare earths is discussed in this document.
-
Citations
Citations to this article as recorded by- Synthesis and magnetic properties of Sm2Co17 particles using salt-assisted spray pyrolysis and a reduction-diffusion process
Tae-Yeon Hwang, Jimin Lee, Min Kyu Kang, Gyutae Lee, Jongryoul Kim, Yong-Ho Choa
Applied Surface Science.2019; 475: 986. CrossRef - Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues
Seo-Ho Shin, Hyun-Ock Kim, Kyung-Taek Rim
Safety and Health at Work.2019; 10(4): 409. CrossRef
- Synthesis and magnetic properties of Sm2Co17 particles using salt-assisted spray pyrolysis and a reduction-diffusion process
- [Korean]
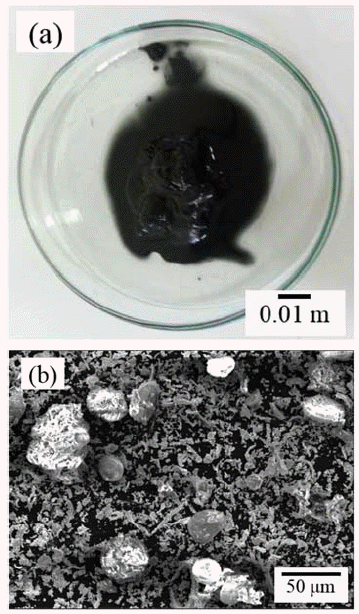
- Recovery of Tungsten from WC/Co Hardmetal Sludge by Alkaline Leaching Hydrometallurgy Process
- Gil-Geun Lee, Ji-Eun Kwon
- J Korean Powder Metall Inst. 2016;23(5):372-378. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.372
- 705 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF This study focuses on the development of an alkaline leaching hydrometallurgy process for the recovery of tungsten from WC/Co hardmetal sludge, and an examination of the effect of the process parameters on tungsten recovery. The alkaline leaching hydrometallurgy process has four stages, i.e., oxidation of the sludge, leaching of tungsten by NaOH, refinement of the leaching solution, and precipitation of tungsten. The WC/Co hardmetal sludge oxide consists of WO3 and CoWO4. The leaching of tungsten is most affected by the leaching temperature, followed by the NaOH concentration and the leaching time. About 99% of tungsten in the WC/Co hardmetal sludge is leached at temperatures above 90°C and a NaOH concentration above 15%. For refinement of the leaching solution, pH control of the solution using HCl is more effective than the addition of Na2S·9H2O. The tungsten is precipitated as high-purity H2WO4·H2O by pH control using HCl. With decreasing pH of the solution, the tungsten recovery rate increases and then decrease. About 93% of tungsten in the WC/Co hardmetal sludge is recovered by the alkaline leaching hydrometallurgy process.
-
Citations
Citations to this article as recorded by- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
Min Soo Park, Jong-Min Gwak, Kyeong-mi Jang, Gook-Hyun Ha
Powder Metallurgy.2023; 66(5): 688. CrossRef
- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
- [Korean]
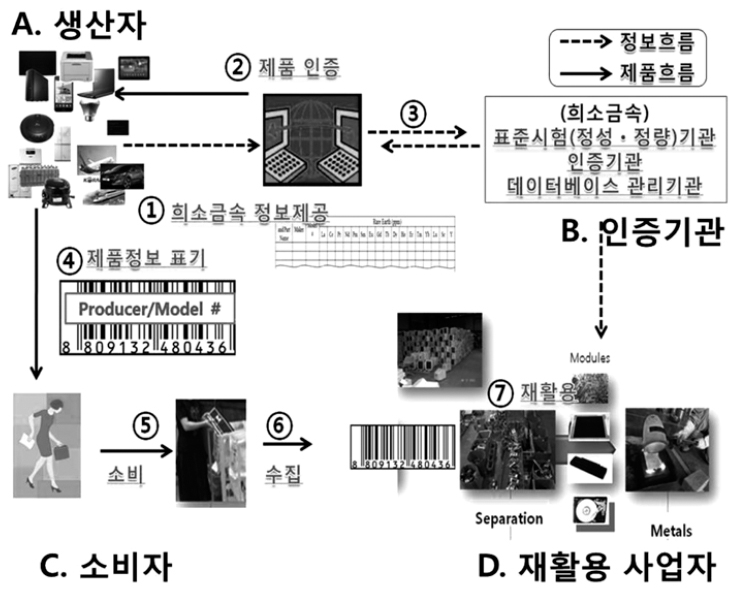
- Status of ITU-T International Standard Development on Rare Metal Recycling
- Mi Hye Lee, Won Jung Choi, Seok-Jun Seo, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(4):325-330. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.325
- 947 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Owing to increasing demand of rare metals present in ICT products, it is necessary to promote the rare metal recycling industry from an environmental viewpoint and to prevent climate change. Despite the fact that information for toxic substances is partly indicated, a legal basis and an international standard indicating usage of rare metals is insufficient. In order to address this issue, a newly created study group of environment and climate change at the ITU (International Telecommunication Union) is doing research to develop methodologies for recycling rare metals from ICT products in an eco-friendly way. Under this group, the Republic of Korea has established two international standards related to rare metals present in ICT products. The first is ‘Release of rare metal information for ICT products (ITU-T L.1100)’ and the other is ‘Quantitative and qualitative analysis methods for rare metals (ITU-T L.1101)’. A new proposal for recommending the provision of rare metal information through a label by manufacturers and consumer/recycling businesses has been approved recently and is supposed to be published later in 2016. Moreover, these recommendations are also being extended to IEC, ISO and other standardization organizations and a strategy to reinforce the ability for domestic standardization is being established in accordance with industrial requirements. This will promote efficient recycling of rare metals from ICT products and will help improve the domestic supply of rare metals.
-
Citations
Citations to this article as recorded by- The State of Affairs of ‘Rare Metal Industry’ in Korea
Jae Hong Shin, Lee Ro Woon, Kyoung Tae Park
Kompleksnoe Ispolʹzovanie Mineralʹnogo syrʹâ/Complex Use of Mineral Resources/Mineraldik Shikisattardy Keshendi Paidalanu.2024; 330(3): 43. CrossRef - Russian Mineral Market Flow and Economic Direction for Securing Stable Resources
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bin Lee, Kyoung Mook Lim, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(4): 345. CrossRef - Trends and Implications of International Standardization for Rare Earths
Sardar Farhat Abbas, Sang-Hyun lee, Bin Lee, Bum-Sung Kim, Taek-Soo Kim
Journal of Korean Powder Metallurgy Institute.2018; 25(2): 165. CrossRef
- The State of Affairs of ‘Rare Metal Industry’ in Korea
- [Korean]
- Influence of Oxidation Temperatures on the Structure and the Microstructure of GaN MOCVD Scraps
- Hyun Seon Hong, Joong Woo Ahn
- J Korean Powder Metall Inst. 2015;22(4):278-282. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.278

- 914 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The GaN-powder scrap generated in the manufacturing process of LED contains significant amounts of gallium. This waste can be an important resource for gallium through recycling of scraps. In the present study, the influence of annealing temperatures on the structural properties of GaN powder was investigated when the waste was recycled through the mechanochemical oxidation process. The annealing temperature varied from 200°C to 1100°C and the changes in crystal structure and microstructure were studied. The annealed powder was characterized using various analytical tools such as TGA, XRD, SEM, and XRF. The results indicate that GaN structure was fully changed to Ga2O3 structure when annealed above 900°C for 2 h. And, as the annealing temperature increased, crystallinity and particle size were enhanced. The increase in particle size of gallium oxide was possibly promoted by powder-sintering which merged particles to larger than 50 nm.
-
Citations
Citations to this article as recorded by- High-temperature thermo-mechanical behavior of functionally graded materials produced by plasma sprayed coating: Experimental and modeling results
Kang Hyun Choi, Hyun-Su Kim, Chang Hyun Park, Gon-Ho Kim, Kyoung Ho Baik, Sung Ho Lee, Taehyung Kim, Hyoung Seop Kim
Metals and Materials International.2016; 22(5): 817. CrossRef
- High-temperature thermo-mechanical behavior of functionally graded materials produced by plasma sprayed coating: Experimental and modeling results
- [Korean]
- Current Technology Trends Analysis on the Recovery of Rare Earth Elements from Fluorescent Substance in the Cold Cathode Fluorescent Lamps of Waste Flat Panel Displays
- Leeseung Kang, Dongyoon Shin, Jieun Lee, Joong Woo Ahn, Hyun-Seon Hong
- J Korean Powder Metall Inst. 2015;22(1):27-31. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.27

- 640 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Flat panel display devices are mainly used as information display devices in the 21st century. The worldwide waste flat panel displays are expected at 2-3 million units but most of them are land-filled for want of a proper recycling technology More specifically, rare earth metals of La and Eu are used as fluorescent materials of Cold Cathode Flourscent Lamp(CCFL)s in the waste flat panel displays and they are critically vulnerable and irreplaceable strategic mineral resources. At present, most of the waste CCFLs are disposed of by land-filling and incineration and proper recovery of 80-plus tons per annum of the rare earth fluorescent materials will significantly contribute to steady supply of them. A dearth of Korean domestic research results on recovery and recycling of rare earth elements in the CCFLs prompts to initiate this status report on overseas research trends and noteworthy research results in related fields.
- [Korean]
- Fabrication of LiNiO2 using NiSO4 Recovered from NCM (Li[Ni,Co,Mn]O2) Secondary Battery Scraps and Its Electrochemical Properties
- Yong-Gyu Kwag, Mi-So Kim, Yoo-Young Kim, Im-Sic Choi, Dong-Kyu Park, In-Sup Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2014;21(4):286-293. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.286

- 713 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The electrochemical properties of cells assembled with the LiNiO2 (LNO) recycled from cathode materials of waste lithium secondary batteries (Li[Ni,Co,Mn]O2), were evaluated in this study. The leaching, neutralization and solvent extraction process were applied to produce high-purity NiSO4 solution from waste lithium secondary batteries. High-purity NiO powder was then fabricated by the heat-treatment and mixing of the NiSO4 solution and H2C2O4. Finally, LiNiO2 as a cathode material for lithium ion secondary batteries was synthesized by heat treatment and mixing of the NiO and Li2CO3 powders. We assembled the cells using the LiNiO2 powders and evaluated the electrochemical properties. Subsequently, we evaluated the recycling possibility of the cathode materials for waste lithium secondary battery using the processes applied in this work.
- [Korean]
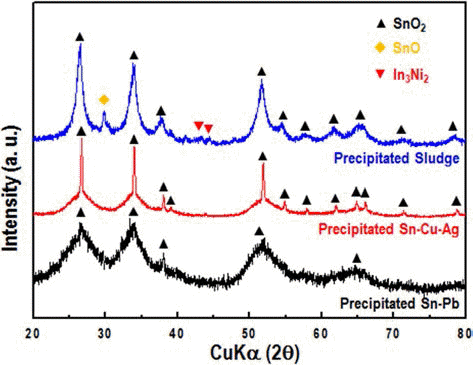
- Development of Pre-treatment for Tin Recovery from Waste Resources
- Y. H Jin, D. H Jang, H. C Jung, K. W Lee
- J Korean Powder Metall Inst. 2014;21(2):142-146. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.142
- 642 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Fundamental experiences have been studied for development of pre-treatment process of Sn by-products such as solders. Dry and wet separation/recovery processes were considered by the differences of physical properties. The by-products, which are analyzed by solder metal and oxides. The metal and oxide were simply separated by dry ball-milling process for 12 hours, after that recovery metal powder might be reusable as lead or lead-free solders. In terms of wet separation process, samples were dissolved in HNO3 + H2O2 and the precipitation were analyzed by SnO2. Overall efficiency of recovery might be increasing via developing simple pre-treatment process.
-
Citations
Citations to this article as recorded by- Material Flow Analysis for Stable Supply and Demand Management of Tin
Sang Hyun Oh, Hong-Yoon Kang, Yong Woo Hwang, Doo Hwan Kim, Kayoung Shin, Nam Seok Kim
Resources Recycling.2023; 32(4): 18. CrossRef - The Current Status and Securing Strategies of tin Resources for Future Core Industries
Hoseok Jeon, Dohyun Jeong, Yeoni Chu, Seongmin Kim
Journal of the Korean Society of Mineral and Energy Resources Engineers.2022; 59(4): 408. CrossRef
- Material Flow Analysis for Stable Supply and Demand Management of Tin
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
- Jae-won Lee, Dae Weon Kim, Seong Tae Jang
- J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
- 550 View
- 3 Download
-
 Abstract
Abstract
 PDF
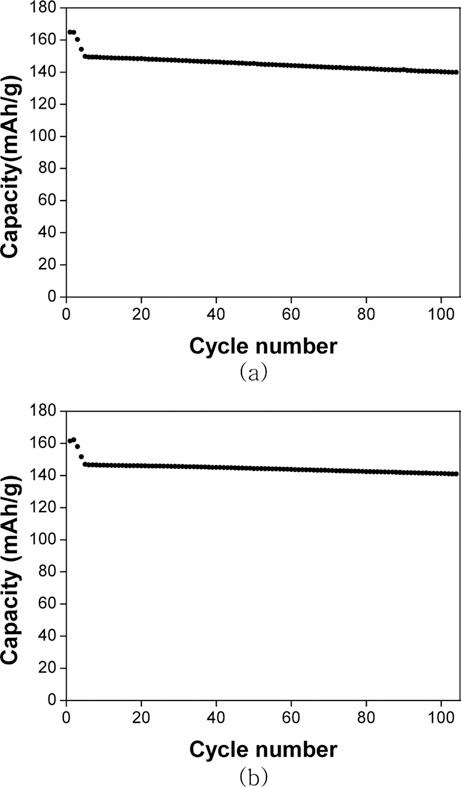
PDF Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
TOP
 KPMI
KPMI


 First
First Prev
Prev


