Search
- Page Path
- HOME > Search
- [Korean]
- Enhanced H2S Gas Sensing Using ZnO Porous Nanorod Synthesized via a Rotational Hydrothermal Method
- Jimyeong Park, Changyu Kim, Minseo Kim, Jiyeon Shin, Jae-Hyoung Lee, Myung Sik Choi
- J Powder Mater. 2025;32(5):406-415. Published online October 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00262
- 416 View
- 7 Download
-
 Abstract
Abstract
 PDF
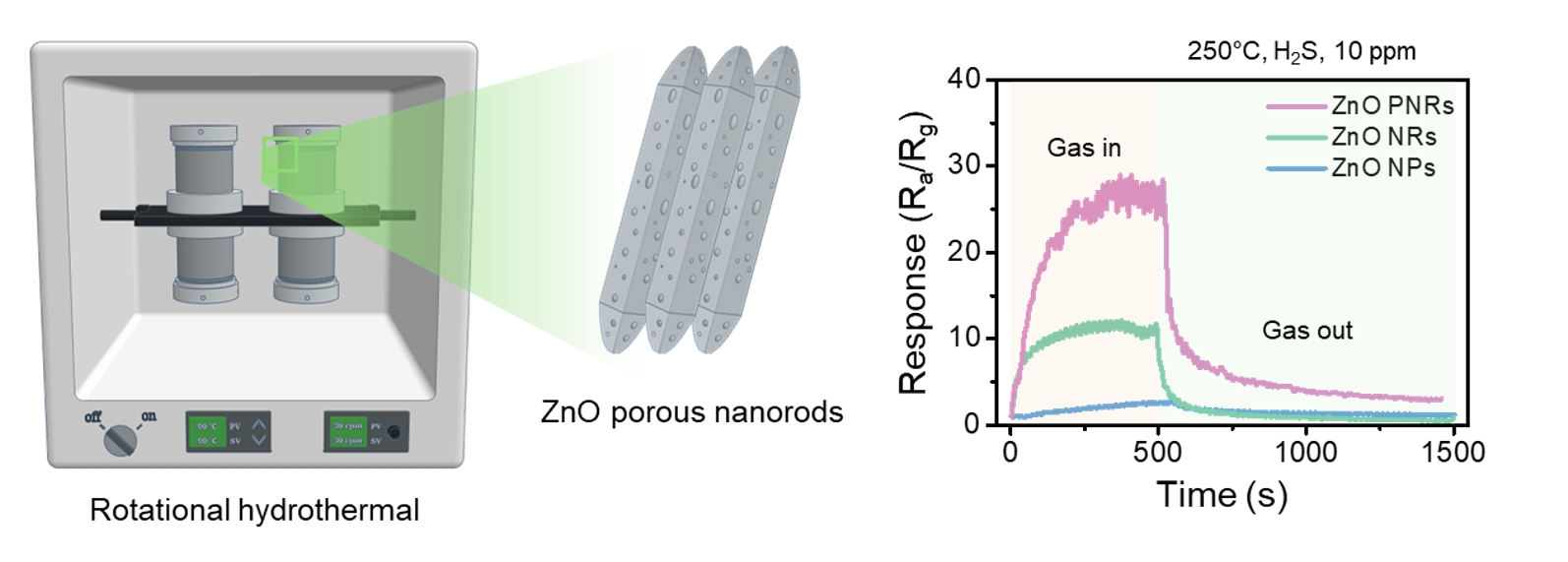
PDF - In this study, ZnO porous nanorods were synthesised using a rotational hydrothermal process, and their performance as hydrogen sulphide (H2S) gas sensors was analysed. Compared to commercial ZnO nanoparticles and conventionally hydrothermally synthesised ZnO nanorods, the ZnO porous nanorods exhibited a more uniform structure and improved crystal growth in the (002) plane, with surfaces rich in porosity and oxygen vacancies. These structural and chemical characteristics significantly improved the sensitivity toward H2S, showing high detection performance at 250°C across various concentrations of H2S gas. Additionally, the sensor demonstrated excellent selectivity against other gases such as C2H5OH, C6H6, C7H8, and NH3. This study indicated that the rotational hydrothermal process is an effective method for developing high-performance ZnO-based gas sensors and suggests its applicability to other metal oxide materials.
- [Korean]
- Fabrication of Polymer Composite with Enhanced Insulation and Mechanical Properties using Aluminum Borate Nanowhiskers
- Junhyeok Choi, Sangin Lee, Kiho Song, Taekyung Kim, Changui Ahn
- J Powder Mater. 2023;30(4):356-362. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.356
- 948 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Inorganic-organic composites find extensive application in various fields, including electronic devices and light-emitting diodes. Notably, encapsulation technologies are employed to shield electronic devices (such as printed circuit boards and batteries) from stress and moisture exposure while maintaining electrical insulation. Polymer composites can be used as encapsulation materials because of their controllable mechanical and electrical properties. In this study, we propose a polymer composite that provides good electrical insulation and enhanced mechanical properties. This is achieved by using aluminum borate nanowhiskers (ABOw), which are fabricated using a facile synthesis method. The ABOw fillers are created via a hydrothermal method using aluminum chloride and boric acid. We confirm that the synthesis occurs in various morphologies based on the molar ratio. Specifically, nanowhiskers are synthesized at a molar ratio of 1:3 and used as fillers in the composite. The fabricated ABOw/epoxy composites exhibit a 48.5% enhancement in mechanical properties, similar to those of pure epoxy, while maintaining good electrical insulation.
-
Citations
Citations to this article as recorded by- Fabrication of Al18B4O33 Spherical Powder with Increased Fluidity via Control of B2O3 Particle Size and Distribution
Kiho Song, Sang in Lee, Hyunseung Song, Changui Ahn
Journal of Powder Materials.2024; 31(6): 513. CrossRef
- Fabrication of Al18B4O33 Spherical Powder with Increased Fluidity via Control of B2O3 Particle Size and Distribution
- [Korean]
- Synthesis and Application of Magnetoplasmonic Nanoparticles
- Sejeong Park, Siyeong Hwang, Seonghwan Jung, Juyong Gwak, Jaebeom Lee
- J Korean Powder Metall Inst. 2021;28(5):429-434. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.429

- 730 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF Magnetic nanoparticles have a significant impact on the development of basic sciences and nanomedical, electronic, optical, and biotech industries. The development of magnetic structures with size homogeneity, magnetization, and particle dispersibility due to high-quality process development can broaden their utilization for separation analysis, structural color optics using surface modification, and energy/catalysts. In addition, magnetic nanoparticles simultaneously exhibit two properties: magnetic and plasmon resonance, which can be self-assembled and can improve signal sensitivity through plasmon resonance. This paper reports typical examples of the synthesis and properties of various magnetic nanoparticles, especially magnetoplasmonic nanoparticles developed in our laboratory over the past decade, and their optical, electrochemical, energy/catalytic, and bio-applications. In addition, the future value of magnetoplasmonic nanoparticles can be reevaluated by comparing them with that reported in the literature.
- [Korean]
- Effect of Hydrothermal Reaction Conditions on Piezoelectric Output Performance of One Dimensional BaTiO3 Nanotube Arrays
- Jae Hoon Lee, Dong Yeol Hyeon, Dong Hun Heo, Kwi-Il Park
- J Korean Powder Metall Inst. 2021;28(2):127-133. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.127
- 983 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF One-dimensional (1D) piezoelectric nanostructures are attractive candidates for energy generation because of their excellent piezoelectric properties attributed to their high aspect ratios and large surface areas. Vertically grown BaTiO3 nanotube (NT) arrays on conducting substrates are intensively studied because they can be easily synthesized with excellent uniformity and anisotropic orientation. In this study, we demonstrate the synthesis of 1D BaTiO3 NT arrays on a conductive Ti substrate by electrochemical anodization and sequential hydrothermal reactions. Subsequently, we explore the effect of hydrothermal reaction conditions on the piezoelectric energy conversion efficiency of the BaTiO3 NT arrays. Vertically aligned TiO2 NT arrays, which act as the initial template, are converted into BaTiO3 NT arrays using hydrothermal reaction with various concentrations of the Ba source and reaction times. To validate the electrical output performance of the BaTiO3 NT arrays, we measure the electricity generated from each NT array packaged with a conductive metal foil and epoxy under mechanical pushings. The generated output voltage signals from the BaTiO3 NT arrays increase with increasing concentration of the Ba source and reaction time. These results provide a new strategy for fabricating advanced 1D piezoelectric nanostructures by demonstrating the correlation between hydrothermal reaction conditions and piezoelectric output performance.
-
Citations
Citations to this article as recorded by- Optimized Process and Mechanical and Electrical Analysis of Polyimide/Pb(Zr,Ti)O3-Based Flexible Piezoelectric Composites
Junki Lee, Sang-il Yoon, Hyunseung Kim, Chang Kyu Jeong
Journal of Powder Materials.2025; 32(1): 16. CrossRef - Fabrication of Flexible Energy Harvester Based on BaTiO3 Piezoelectric Nanotube Arrays
Seo Young Yoon, Cheol Min Kim, Bitna Bae, Yujin Na, Haksu Jang, Kwi-Il Park
journal of Korean Powder Metallurgy Institute.2023; 30(6): 521. CrossRef
- Optimized Process and Mechanical and Electrical Analysis of Polyimide/Pb(Zr,Ti)O3-Based Flexible Piezoelectric Composites
- [Korean]
- Effect of H2SO4 and Reaction Time on Synthesis of 5Mg(OH)2∙MgSO4∙3H2O Whiskers using Hydrothermal Reaction
- Areum Choi, Nuri Oh, YooJin Kim
- J Korean Powder Metall Inst. 2020;27(5):401-405. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.401

- 1,036 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Magnesium hydroxide sulfate hydrate (MHSH) whiskers were synthesized via a hydrothermal reaction by using MgO as the reactant as well as the acid solution. The effects of the H2SO4 amount and reaction time at the same temperature were studied. In general, MHSH whiskers were prepared using MgSO4 in aqueous ammonia. In this work, to reduce the formation of impurities and increase the purity of MHSH, we employed a synthesis technique that did not require the addition of a basic solution. Furthermore, the pH value, which was controlled by the H2SO4 amount, acted as an important factor for the formation of high-purity MHSH. MgO was used as the raw material because it easily reacts in water and forms Mg+ and MgOH+ ions that bind with SO4 2- ions to produce MHSH. Their morphologies and structures were determined using X-ray diffraction (XRD) and scanning electron microscopy (SEM).
-
Citations
Citations to this article as recorded by- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
Journal of Powder Materials.2025; 32(5): 399. CrossRef - Study of SiO2 coating and carboxylic surface-modification on Mg-based inorganic fiber by one-step reflux reaction
Minsol Park, Areum Choi, Seiki Kim, Wooyoung Shim, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(6): 869. CrossRef - Effect of sulfate ion on synthesis of 5 Mg(OH)2·MgSO4·3H2O whiskers using non-hydrothermal method with acid catalyst
Areum Choi, Nuri Oh, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(2): 224. CrossRef
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
- [Korean]
- Magnetic Properties of Micron Sized Fe3O4 Crystals Synthesized by Hydrothermal Methods
- Ki-Bum Lee, Chunghee Nam
- J Korean Powder Metall Inst. 2019;26(6):481-486. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.481
- 1,150 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF Iron oxides currently attract considerable attention due to their potential applications in the fields of lithiumion batteries, bio-medical sensors, and hyperthermia therapy materials. Magnetite (Fe3O4) is a particularly interesting research target due to its low cost, good biocompatibility, outstanding stability in physiological conditions. Hydrothermal synthesis is one of several liquid-phase synthesis methods with water or an aqueous solution under high pressure and high temperature. This paper reports the growth of magnetic Fe3O4 particles from iron powder (spherical, <10 μm) through an alkaline hydrothermal process under the following conditions: (1) Different KOH molar concentrations and (2) different synthesis time for each KOH molar concentrations. The optimal condition for the synthesis of Fe3O4 using Fe powders is hydrothermal oxidation with 6.25 M KOH for 48 h, resulting in 89.2 emu/g of saturation magnetization at room temperature. The structure and morphologies of the synthesized particles are characterized by X-ray diffraction (XRD, 2θ = 20°–80°) with Cu-kα radiation and field emission scanning electron microscopy (FE-SEM), respectively. The magnetic properties of magnetite samples are investigated using a vibrating sample magnetometer (VSM). The role of KOH in the formation of magnetite octahedron is observed.
- [Korean]
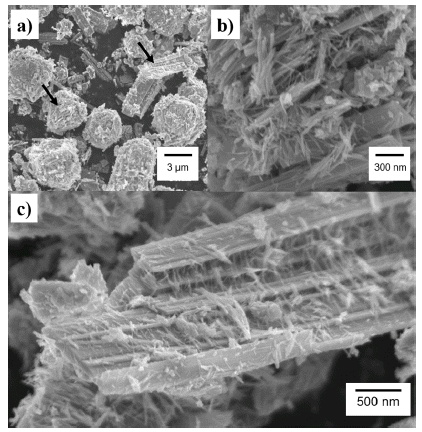
- Fabrication and Characterization of Hexagonal Tungsten Oxide Nanopowders for High Performance Gas Sensing Application
- Jinsoo Park
- J Korean Powder Metall Inst. 2019;26(1):28-33. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.28
- 834 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF The gas sensor is essential to monitoring dangerous gases in our environment. Metal oxide (MO) gas sensors are primarily utilized for flammable, toxic and organic gases and O3 because of their high sensitivity, high response and high stability. Tungsten oxides (WO3) have versatile applications, particularly for gas sensor applications because of the wide bandgap and stability of WO3. Nanosize WO3 are synthesized using the hydrothermal method. Asprepared WO3 nanopowders are in the form of nanorods and nanorulers. The crystal structure is hexagonal tungsten bronze (MxWO3, x =< 0.33), characterized as a tunnel structure that accommodates alkali ions and the phase stabilizer. A gas detection test reveals that WO3 can detect acetone, butanol, ethanol, and gasoline. This is the first study to report this capability of WO3.
- [Korean]
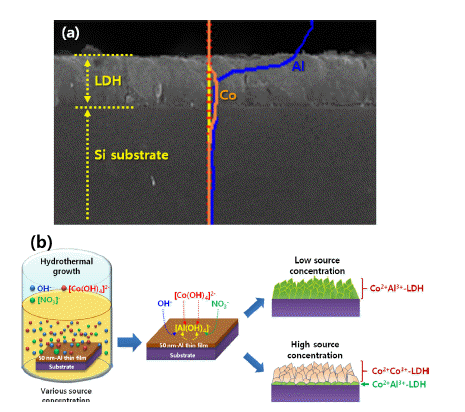
- Recent Development in Fabrication and Control of Layered-Double Hydroxide Nanostructures
- Chan-Woo Jeon, Il-Kyu Park
- J Korean Powder Metall Inst. 2018;25(6):514-522. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.514
- 1,848 View
- 25 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Layered-double hydroxide (LDH)-based nanostructures offer the two-fold advantage of being active catalysts with incredibly large specific surface areas. As such, they have been studied extensively over the last decade and applied in roles as diverse as light source, catalyst, energy storage mechanism, absorber, and anion exchanger. They exhibit a unique lamellar structure consisting of a wide variety of combinations of metal cations and various anions, which determine their physical and chemical performances, and make them a popular research topic. Many reviewed papers deal with these unique properties, synthetic methods, and applications. Most of them, however, are focused on the form-factor of nanopowder, as well as on the control of morphologies via one-step synthetic methods. LDH nanostructures need to be easy to control and fabricate on rigid substrates such as metals, semiconductors, oxides, and insulators, to facilitate more viable applications of these nanostructures to various solid-state devices. In this review, we explore ways to grow and control the various LDH nanostructures on rigid substrates.
-
Citations
Citations to this article as recorded by- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
Seon Yong Lee, YoungJae Kim, Young Jae Lee
Economic and Environmental Geology.2023; 56(1): 23. CrossRef
- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
- [Korean]
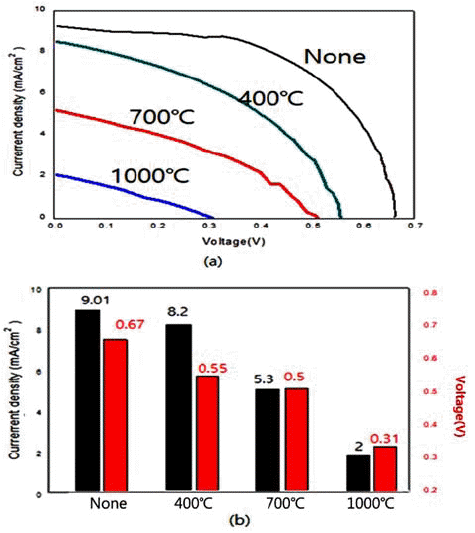
- Synthesis of Nanopowders by Hydrothermal Method and their Application to Dye-sentisized Solar Cell Materials
- JinYoung Lim, Jeongseok Ahn, Jung-Ho Ahn
- J Korean Powder Metall Inst. 2018;25(4):309-315. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.309
- 680 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In the present work, we synthesize nano-sized ZnO, SnO2, and TiO2 powders by hydrothermal reaction using metal chlorides. We also examine the energy-storage characteristics of the resulting materials to evaluate the potential application of these powders to dye-sensitized solar cells. The control of processing parameters such as pressure, temperature, and the concentration of aqueous solution results in the formation of a variety of powder morphologies with different sizes. Nano-rod, nano-flower, and spherical powders are easily formed with the present method. Heat treatment after the hydrothermal reaction usually increases the size of the powder. At temperatures above 1000°C, a complete collapse of the shape occurs. With regard to the capacity of DSSC materials, the hydrothermally synthesized TiO2 results in the highest current density of 9.1 mA/cm2 among the examined oxides. This is attributed to the fine particle size and morphology with large specific surface area.
- [Korean]
- Study on preparation and photocatalytic properties of F-containing TiO2 nanopowders using wet-process from Ammonium Hexafluorotitanate
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Hyeon-Mo Kim, Kyung-Soo Park
- J Korean Powder Metall Inst. 2018;25(3):226-331. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.226

- 524 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF F-containing TiO2 nanopowders are synthesized using simple wet processes (precipitation-based and hydrothermal) from ammonium hexafluorotitanate (AHFT, (NH4)2TiF6) as a precursor to apply as a photocatalyst for the degradation of rhodamine B (RhB). The surface properties of the prepared samples are evaluated using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). The results confirm that the synthesized anatase TiO2 has sphere-like shapes, with numerous small nanoparticles containing fluorine on the surface. The photocatalytic activity of F-containing TiO2 compared with F-free TiO2 is characterized by measuring the degradation of RhB using a xenon lamp. The photocatalytic degradation of F-containing TiO2 exhibits improved photocatalytic activity, based on the positive effects of adsorbed F ions on the surface.
- [Korean]
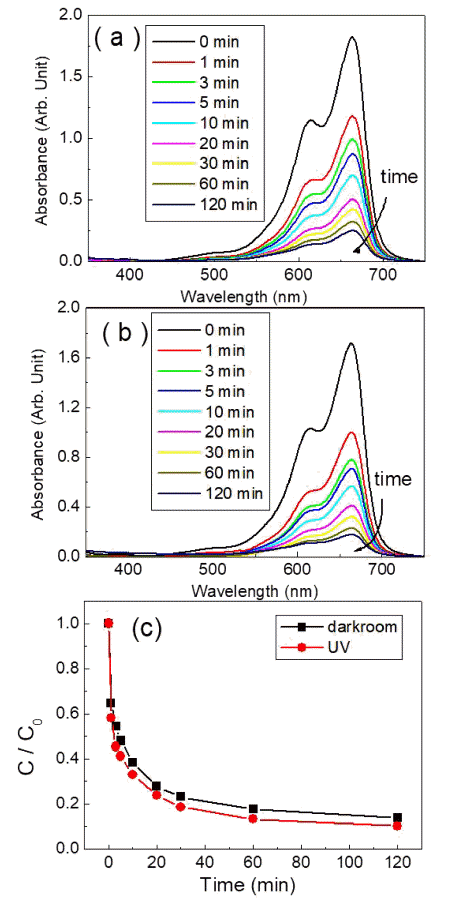
- Photocatalytic and Adsorption Properties of WO3 Nanorods Prepared by Hydrothermal Synthesis
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2017;24(6):483-488. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.483
- 1,146 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Transition-metal oxide semiconductors have various band gaps. Therefore, many studies have been conducted in various application fields. Among these, methods for the adsorption of organic dyes and utilization of photocatalytic properties have been developed using various metal oxides. In this study, the adsorption and photocatalytic effects of WO3 nanomaterials prepared by hydrothermal synthesis are investigated, with citric acid added in the hydrothermal process as a structure-directing agent. The nanostructures of WO3 are studied using transmission electron microscopy and scanning electron microscopy images. The crystal structure is investigated using X-ray diffraction patterns, and the changes in the dye concentrations adsorbed on WO3 nanorods are measured with a UV-visible absorption spectrophotometer based on Beer-Lambert’s law. The methylene blue (MB) dye solution is subjected to acid or base conditions to monitor the change in the maximum adsorption amount in relation to the pH. The maximum adsorption capacity is observed at pH 3. In addition to the dye adsorption, UV irradiation is carried out to investigate the decomposition of the MB dye as a result of photocatalytic effects. Significant photocatalytic properties are observed and compared with the adsorption effects for dye removal.
-
Citations
Citations to this article as recorded by- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
Kwang-Mo Kang, Ji-Hye Jeong, Ga-In Lee, Jae-Min Im, Hyun-Jeong Cheon, Deok-Hyeon Kim, Yoon-Chae Nah
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 40. CrossRef - Photocatalysis of TiO<sub>2</sub>/WO<sub>3</sub> Composites Synthesized by Ball Milling
Su-Yeol Yu, Chunghee Nam
Journal of Korean Powder Metallurgy Institute.2018; 25(4): 316. CrossRef
- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
- [English]
- The Synthesis and Photocatalytic activity of Carbon Nanotube-mixed TiO2 Nanotubes
- Chun Woong Park, Young Do Kim, Tohru Sekino, Se Hoon Kim
- J Korean Powder Metall Inst. 2017;24(4):279-284. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.279
- 1,517 View
- 11 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
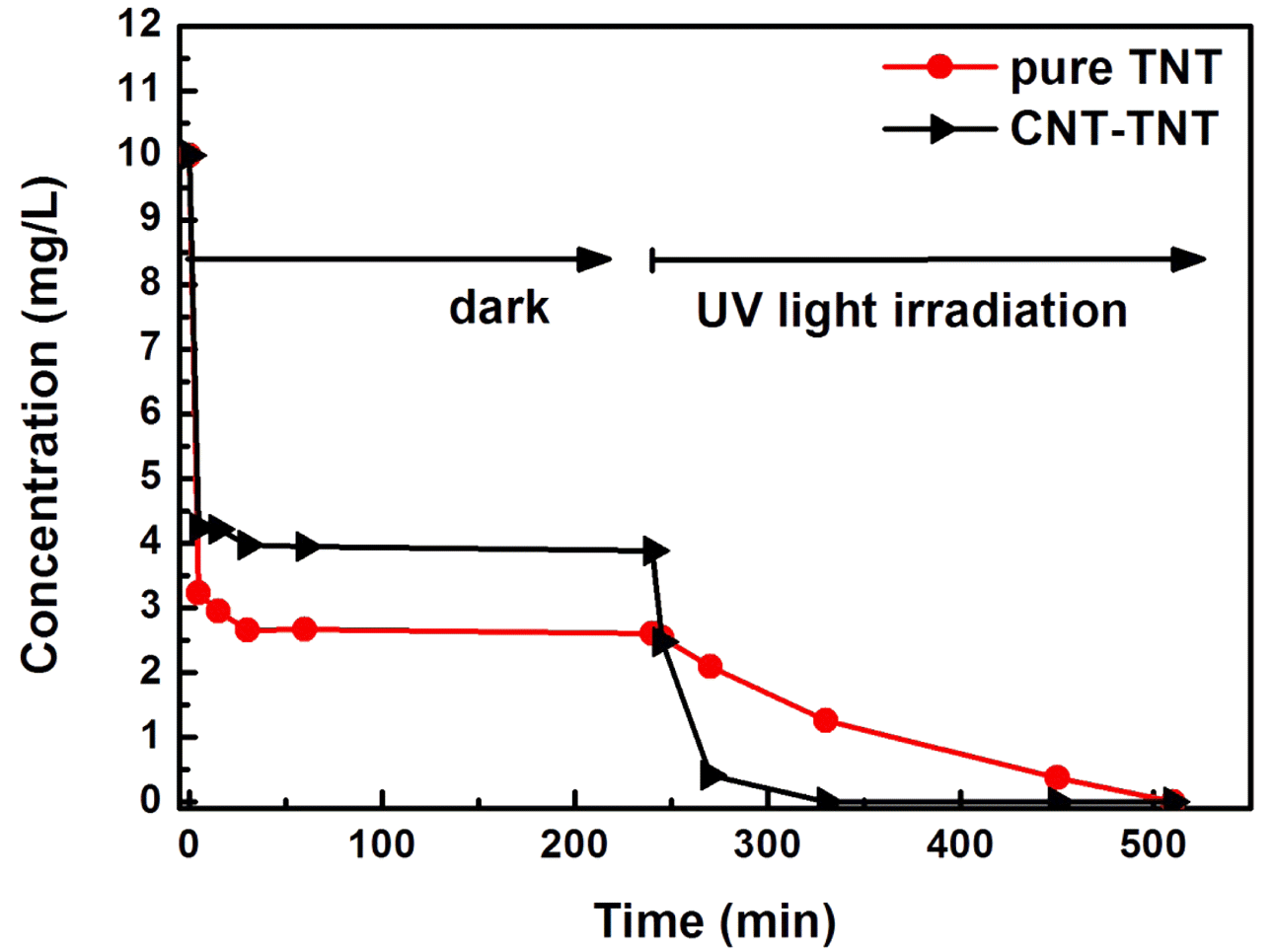
PDF The formation mechanism and photocatalytic properties of a multiwalled carbon nanotube (MWCNT)/TiO2- based nanotube (TNTs) composite are investigated. The CNT/TNT composite is synthesized via a solution chemical route. It is confirmed that this 1-D nanotube composite has a core-shell nanotubular structure, where the TNT surrounds the CNT core. The photocatalytic activity investigated based on the methylene blue degradation test is superior to that of with pure TNT. The CNTs play two important roles in enhancing the photocatalytic activity. One is to act as a template to form the core-shell structure while titanate nanosheets are converted into nanotubes. The other is to act as an electron reservoir that facilitates charge separation and electron transfer from the TNT, thus decreasing the electronhole recombination efficiency.
-
Citations
Citations to this article as recorded by- Carbon-based photocatalysis in organic transformation
Md Razu Ahmed, Yuta Nishina
Bulletin of the Chemical Society of Japan.2026;[Epub] CrossRef - Low-Dimensional Carbon and Titania Nanotube Composites via a Solution Chemical Process and Their Nanostructural and Electrical Properties for Electrochemical Devices
Sunghun Eom, Sung Hun Cho, Tomoyo Goto, Myoung Pyo Chun, Tohru Sekino
ACS Applied Nano Materials.2019; 2(10): 6230. CrossRef
- Carbon-based photocatalysis in organic transformation
- [Korean]
- Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
- Il-Kyu Park
- J Korean Powder Metall Inst. 2016;23(4):270-275. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.270
- 874 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
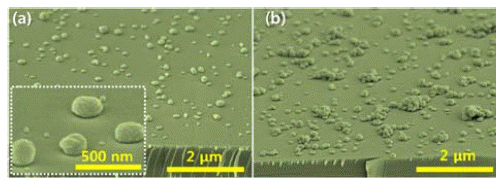
PDF We report on an all-solution-processed hydrothermal method to control the morphology of ZnO nanostructures on Si substrates from three-dimensional hemispherical structures to two-dimensional thin film layers, by controlling the seed layer and the molar contents of surfactants during their primary growth. The size and the density of the seed layer, which is composed of ZnO nanodots, change with variation in the solute concentration. The ZnO nanodots act as heterogeneous nucleation sites for the main ZnO nanostructures. When the seed layer concentration is increased, the ZnO nanostructures change from a hemispherical shape to a thin film structure, formed by densely packed ZnO hemispheres. In addition, the morphology of the ZnO layer is systematically controlled by using trisodium citrate, which acts as a surfactant to enhance the lateral growth of ZnO crystals rather than a preferential one-dimensional growth along the c-direction. X-ray diffraction and energy dispersive X-ray spectroscopy results reveal that the ZnO structure is wurtzite and did not incorporate any impurities from the surfactants used in this study.
-
Citations
Citations to this article as recorded by- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
Leeseung Kang, HyeLan An, Ji Young Park, Myung Hwan Hong, Sahn Nahm, Chan Gi Lee
Applied Surface Science.2019; 475: 969. CrossRef
- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
- [Korean]
- Fabrication of ZnO Nanorod/polystyrene Nanosphere Hybrid Nanostructures by Hydrothermal Method for Energy Generation Applications
- Seong-Ho Baek, Il-Kyu Park
- J Korean Powder Metall Inst. 2015;22(6):391-395. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.391

- 965 View
- 3 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF We report on the successful fabrication of ZnO nanorod (NR)/polystyrene (PS) nanosphere hybrid nanostructure by combining drop coating and hydrothermal methods. Especially, by adopting an atomic layer deposition method for seed layer formation, very uniform ZnO NR structure is grown on the complicated PS surfaces. By using zinc nitrate hexahydrate [Zn(NO3)2 ·6H2O] and hexamine [(CH2)6N4] as sources for Zn and O in hydrothermal process, hexagonal shaped single crystal ZnO NRs are synthesized without dissolution of PS in hydrothermal solution. X-ray diffraction results show that the ZnO NRs are grown along c-axis with single crystalline structure and there is no trace of impurities or unintentionally formed intermetallic compounds. Photoluminescence spectrum measured at room temperature for the ZnO NRs on flat Si and PS show typical two emission bands, which are corresponding to the band-edge and deep level emissions in ZnO crystal. Based on these structural and optical investigations, we confirm that the ZnO NRs can be grown well even on the complicated PS surface morphology to form the chestnut-shaped hybrid nanostructures for the energy generation and storage applications.
-
Citations
Citations to this article as recorded by- Synthesis of Planar-Type ZnO Powder in Non-Nano Scale Dimension and Its Application in Ultraviolet Protection Cosmetics
Jung-Hwan Lee, Gun-Sub Lee, Eung-Nam Park, Dong-Hyeon Jo, So-Won Kim, Hee-Chul Lee
Materials.2023; 16(5): 2099. CrossRef - Rapid consolidation of nanostuctured WC-FeAl3 by pulsed current activated heating and its mechanical properties
In-Jin Shon, Seok-Jae Lee
International Journal of Refractory Metals and Hard Materials.2017; 65: 69. CrossRef - Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
Young-Tae Kwon, Sung-Oong Kang, Ji-Ae Cheon, Yoseb Song, Jong-Jin Lee, Yong-Ho Choa
Applied Surface Science.2017; 415: 2. CrossRef - Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 270. CrossRef
- Synthesis of Planar-Type ZnO Powder in Non-Nano Scale Dimension and Its Application in Ultraviolet Protection Cosmetics
- [Korean]
- Synthesis of Perforated Polygonal Cobalt Oxides using a Carbon Nanofiber Template
- Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(5):350-355. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.350
- 826 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
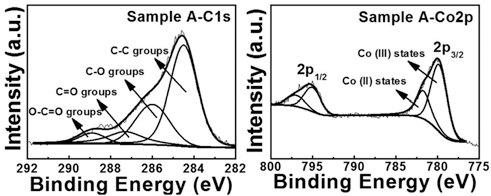
PDF Perforated polygonal cobalt oxide (CO3O4) is synthesized using electrospinning and a hydrothermal method followed by the removal of a carbon nanofiber (CNF) template. To investigate their formation mechanism, thermogravimetric analysis, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and Xray photoelectron spectroscopy are examined. To obtain the optimum condition of perforated polygonal CO3O4, we prepare three different weight ratios of the Co precursor and the CNF template: sample A (Co precursor:CNF template- 10:1), sample B (Co precursor:CNF template-3.2:1), and sample C (Co precursor:CNF template-2:1). Among them, sample A exhibits the perforated polygonal CO3O4 with a thin carbon layer (5.7-6.2 nm) owing to the removal of CNF template. However, sample B and sample C synthesized perforated round CO3O4 and destroyed CO3O4 powders, respectively, due to a decreased amount of Co precursor. The increased amount of the CNF template prevents the formation of polygonal CO3O4. For sample A, the optimized weight ratio of the Co precursor and CNF template may be related to the successful formation of perforated polygonal CO3O4. Thus, perforated polygonal CO3O4 can be applied to electrode materials of energy storage devices such as lithium ion batteries, supercapacitors, and fuel cells.
-
Citations
Citations to this article as recorded by- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef - Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef
- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
- [Korean]
- Fabrication of ZnO Nanorod based Robust Nanogenerator Metal Substrate
- Seong-Ho Baek, Il-Kyu Park
- J Korean Powder Metall Inst. 2015;22(5):331-336. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.331

- 1,249 View
- 6 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF We report on the succesful fabrication of ZnO nanorod (NR)-based robust piezoelectric nanogenerators (PNGs) by using Cu foil substrate. The ZnO NRs are successfully grown on the Cu foil substrate by using all solution based method, a two step hydrothermal synthesis. The ZnO NRs are grown along c-axis well with an average diameter of 75~80 nm and length of 1~1.5 μm. The ZnO NRs showed abnormal photoluminescence specrta which is attributed from surface plasmon resonance assistant enhancement at specific wavelength. The PNGs on the SUS substrates show typical piezoelectric output performance which showing a frequency dependent voltage enhancement and polarity dependent charging and discharging characteristics. The output voltage range is 0.79~2.28 V with variation of input strain frequency of 1.8~3.9 Hz. The PNG on Cu foil shows reliable output performance even at the operation over 200 times without showing degradation of output voltage. The current output from the PNG is 0.7 μA/cm2 which is a typical output range from the ZnO NR-based PNGs. These performance enhancement is attributed from the high flexibility, high electrical conductivity and excellent heat dissipation properties of the Cu foil as a substrate.
-
Citations
Citations to this article as recorded by- Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
Young-Tae Kwon, Sung-Oong Kang, Ji-Ae Cheon, Yoseb Song, Jong-Jin Lee, Yong-Ho Choa
Applied Surface Science.2017; 415: 2. CrossRef - Fabrication of Porous Polytetrafluoroethylene thin Film from Powder Dispersion-solution for Energy Nanogenerator Applications
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 102. CrossRef - Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 270. CrossRef - Fabrication of ZnO Nanorod/polystyrene Nanosphere Hybrid Nanostructures by Hydrothermal Method for Energy Generation Applications
Seong-Ho Baek, Il-Kyu Park
Journal of Korean Powder Metallurgy Institute.2015; 22(6): 391. CrossRef
- Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties
- [Korean]
- Formation of Nano-oxides on Porous Metallic Glass Compacts using Hydrothermal Synthesis
- H. J. Park, Y. S. Kim, S. H. Hong, J. T. Kim, J. Y. Cho, W. H. Lee, K. B. Kim
- J Korean Powder Metall Inst. 2015;22(4):229-233. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.229

- 755 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous metallic glass compact (PMGC) are developed by electro-discharge sintering (EDS) process of gas atomized Zr41.2Ti13.8Cu12.5Ni10Be22.5 metallic glass powder under of 0.2 kJ generated by a 450 μF capacitor being charged to 0.94 kV. Functional iron-oxides are formed and growth on the surface of PMGCs via hydrothermal synthesis. It is carried out at 150°C for 48hr with distilled water of 100 mL containing Fe ions of 0.18 g/L. Consequently, two types of iron oxides with different morphology which are disc-shaped Fe2O3 and needle-shaped Fe3O4 are successfully formed on the surface of the PMGCs. This finding suggests that PMGC witih hydrothermal technique can be attractive for the practical technology as a new area of structural and functional materials. And they provide a promising road map for using the metallic glasses as a potential functional application.
-
Citations
Citations to this article as recorded by- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
- [Korean]
- Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
- Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(5):360-365. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.360
- 725 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
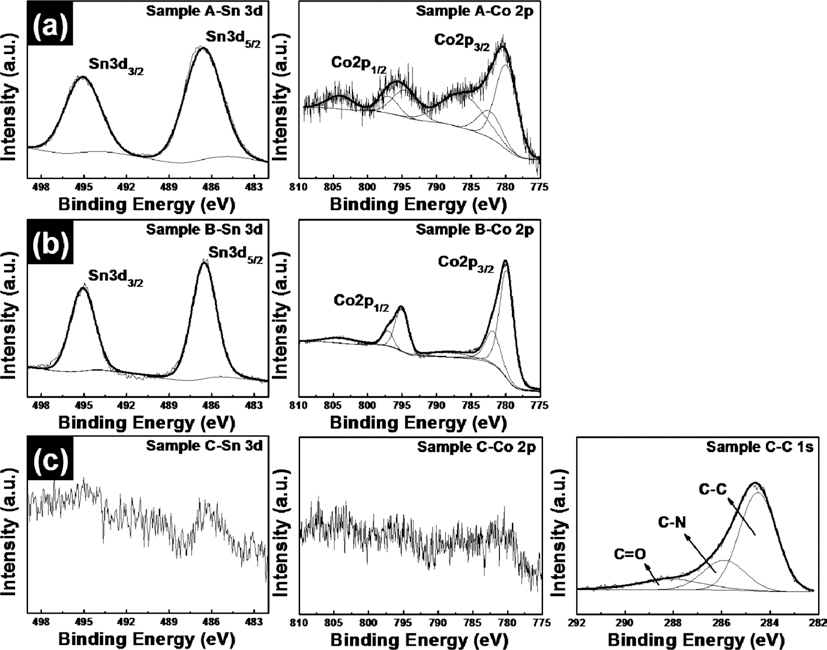
PDF SnO2-CoO/carbon-coated CoO core/shell nanowire composites were synthesized by using electrospinning and hydrothermal methods. In order to obtain SnO2-CoO/carbon-coated CoO core/shell nanowire composites, SnO2-Co3O4 nanowire composites and SnO2-Co3O4/polygonal Co3O4 core/shell nanowire composites are also synthesized. To demonstrate their structural, chemical bonding, and morphological properties, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. These results indicated that the morphologies and structures of the samples were changed from SnO2-Co3O4 nanowires having cylindrical structures to SnO2-Co3O4/Co3O4 core/shell nanowires having polygonal structures after a hydrothermal process. At last, SnO2-CoO/carbon-coated CoO core/shell nanowire composites having irregular and high surface area are formed after carbon coating using a polypyrrole (PPy). Also, there occur phases transformation of cobalt phases from Co3O4 to CoO during carbon coating using a PPy under a argon atmosphere.
-
Citations
Citations to this article as recorded by- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
혜란 안, 혜린 강, 효정 선, 지호 한, 효진 안
Korean Journal of Materials Research.2015; 25(12): 672~677. CrossRef - Synthesis of Perforated Polygonal Cobalt Oxides usinga Carbon Nanofiber Template
Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2015; 22(5): 350. CrossRef
- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
TOP
 KPMI
KPMI


 First
First Prev
Prev


