Search
- Page Path
- HOME > Search
- [English]
- Enhanced Compressive Strength of Fired Iron Ore Pellets: Effects of Blending Fine and Coarse Particle Concentrates
- Ngo Quoc Dung, Tran Xuan Hai, Nguyen Minh Thuyet, Nguyen Quang Tung, Arvind Barsiwal, Nguyen Hoang Viet
- J Powder Mater. 2025;32(4):315-329. Published online August 29, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00129
- 1,811 View
- 70 Download
-
 Abstract
Abstract
 PDF
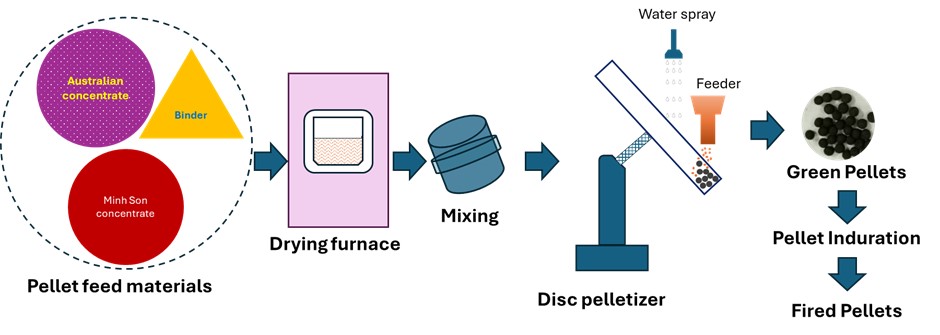
PDF - This study investigated the effects of oxidative firing parameters and raw material characteristics on the pelletization of Australian and Minh Son (Vietnam) iron ore concentrates. The influence of firing temperature (1050°C–1150°C) and holding time (15–120 min) on pellet compressive strength was examined, focusing on microstructural changes during consolidation. Green pellets were prepared using controlled particle size distributions and bentonite as a binder. Scanning electron microscopy and energy-dispersive X-ray spectroscopy analyses revealed that grain boundary diffusion, liquid phase formation, and densification significantly improved mechanical strength. X-ray diffraction confirmed the complete oxidation of magnetite to hematite at elevated temperatures, a critical transformation for metallurgical performance. Optimal firing conditions for both single and blended ore compositions yielded compressive strengths above 250 kgf/pellet, satisfying the requirements for blast furnace applications. These results provide valuable guidance for improving pellet production, promoting the efficient utilization of diverse ore types, and enhancing the overall performance of ironmaking operations.
- [English]
- High-Temperature Steam Oxidation Behavior of Silicide- or Aluminide- Coated Mo and Nb Refractory Metals
- Woojin Lim, Je-Kyun Baek, JaeJoon Kim, Hyun Gil Kim, Ho Jin Ryu
- J Powder Mater. 2024;31(6):546-555. Published online December 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00381
- 1,458 View
- 22 Download
-
 Abstract
Abstract
 PDF
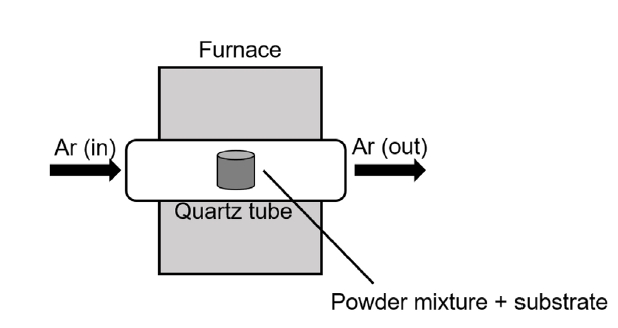
PDF - Refractory materials, such as molybdenum and niobium, are potential candidates for cladding material due to their high melting temperatures and desirable mechanical properties at higher temperatures than those of zirconium alloys. However, refractory materials have low resistance to oxidation at elevated temperatures. Therefore, this study examined silicide or aluminide surface coatings as protection against rapid oxidation of refractory materials at elevated temperatures for a potential accident-tolerant fuel cladding. Silicide or aluminide layers were formed on refractory metal substrates by using the pack cementation method. The steam oxidation behavior of both coated and uncoated samples was compared by thermogravimetric analysis at 1200°C. The weight changes of the coated samples were greatly reduced than those of uncoated samples. Microstructural analyses demonstrated that the silicide and aluminide layers were oxidized to form a protective surface oxide that prevented rapid oxidation of the refractory substrate at elevated temperatures.
- [Korean]
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- Minsu Kim, Hansung Lee, Byungmin Ahn
- J Powder Mater. 2023;30(6):478-483. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.478
- 1,205 View
- 15 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
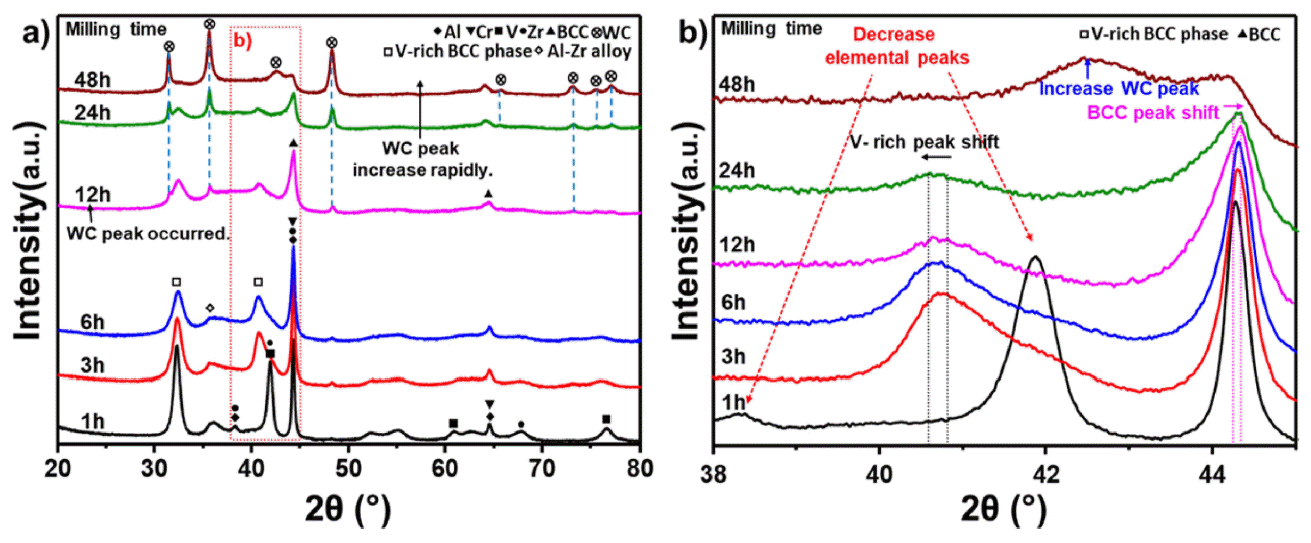
PDF High-entropy alloys (HEAs) are characterized by having five or more main elements and forming simple solids without forming intermetallic compounds, owing to the high entropy effect. HEAs with these characteristics are being researched as structural materials for extreme environments. Conventional refractory alloys have excellent hightemperature strength and stability; however, problems occur when they are used extensively in a high-temperature environment, leading to reduced fatigue properties due to oxidation or a limited service life. In contrast, refractory entropy alloys, which provide refractory properties to entropy alloys, can address these issues and improve the hightemperature stability of the alloy through phase control when designed based on existing refractory alloy elements. Refractory high-entropy alloys require sufficient milling time while in the process of mechanical alloying because of the brittleness of the added elements. Consequently, the high-energy milling process must be optimized because of the possibility of contamination of the alloyed powder during prolonged milling. In this study, we investigated the hightemperature oxidation behavior of refractory high-entropy alloys while optimizing the milling time.
-
Citations
Citations to this article as recorded by- Tailored high-temperature oxidation behavior and nanomechanical properties of Al0.75VCrZrNb lightweight refractory high-entropy alloys
Hansung Lee, Hwi Geun Yu, Reliance Jain, Man Mohan, Younggeon Lee, Sheetal Kumar Dewangan, Byungmin Ahn
International Journal of Refractory Metals and Hard Materials.2026; 135: 107507. CrossRef - Simultaneous enhancement of strength and ductility of Al matrix composites enabled by submicron-sized high-entropy alloy phases
Chahee Jung, Seungin Nam, Hansol Son, Juyeon Han, Jaewon Jeong, Hyokyung Sung, Hyoung Seop Kim, Seok Su Sohn, Hyunjoo Choi
Journal of Materials Research and Technology.2024; 33: 1470. CrossRef
- Tailored high-temperature oxidation behavior and nanomechanical properties of Al0.75VCrZrNb lightweight refractory high-entropy alloys
- [Korean]
- Oxidation Behaviors and Degradation Properties of Aluminide Coated Stainless Steel at High Temperature
- Cheol Hong Hwang, Hyo Min Lee, Jeong Seok Oh, Dong Hyeon Hwang, Yu Seok Hwang, Jong Won Lee, Jeong Mook Choi, Joon Sik Park
- J Korean Powder Metall Inst. 2021;28(5):396-402. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.396
- 752 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
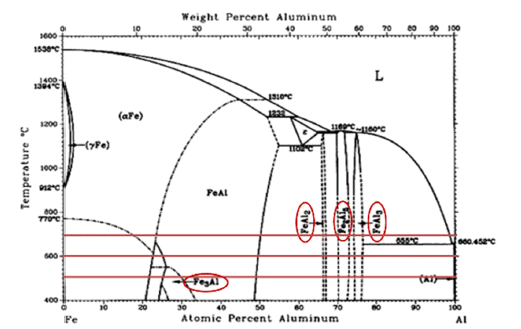
PDF Stainless steel, a type of steel used for high-temperature parts, may cause damage when exposed to high temperatures, requiring additional coatings. In particular, the Cr2O3 product layer is unstable at 1000°C and higher temperatures; therefore, it is necessary to improve the oxidation resistance. In this study, an aluminide (Fe2Al5 and FeAl3) coating layer was formed on the surface of STS 630 specimens through Al diffusion coatings from 500°C to 700°C for up to 25 h. Because the coating layers of Fe2Al5 and FeAl3 could not withstand temperatures above 1200°C, an Al2O3 coating layer is deposited on the surface through static oxidation treatment at 500°C for 10 h. To confirm the ablation resistance of the resulting coating layer, dynamic flame exposure tests were conducted at 1350°C for 5–15 min. Excellent oxidation resistance is observed in the coated base material beneath the aluminide layer. The conditions of the flame tests and coating are discussed in terms of microstructural variations.
-
Citations
Citations to this article as recorded by- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
C. Hwang, J. Park, J. Oh, D. Han, S. Lee, K. Shin, J. Choi, K. P. Shinde, J. S. Park
Metals and Materials International.2023; 29(7): 1855. CrossRef
- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
- [English]
- Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process
- Sangmin Park, Sun-Woo Nam, Sang-Hoon Lee, Myung-Suk Song, Taek-Soo Kim
- J Korean Powder Metall Inst. 2021;28(2):91-96. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.91

- 1,763 View
- 21 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF Rare earth magnets with excellent magnetic properties are indispensable in the electric device, wind turbine, and e-mobility industries. The demand for the development of eco-friendly recycling techniques has increased to realize sustainable green technology, and the supply of rare earth resources, which are critical for the production of permanent magnets, are limited. Liquid metal extraction (LME), which is a type of pyrometallurgical recycling, is known to selectively extract the metal forms of rare earth elements. Although several studies have been carried out on the formation of intermetallic compounds and oxides, the effect of oxide formation on the extraction efficiency in the LME process remains unknown. In this study, microstructural and phase analyses are conducted to confirm the oxidation behavior of magnets pulverized by a jaw crusher. The LME process is performed with pulverized scrap, and extraction percentages are calculated to confirm the effect of the oxide phases on the extraction of Dy during the reaction. During the LME p rocess, Nd i s completely e xtracted a fter 6 h, w hile D y remains as D y2Fe17 and Dy-oxide. Because the decomposition rate of Dy2Fe17 is faster than the reduction rate of Dy-oxide, the importance of controlling Dy-oxide on Dy extraction is confirmed.
-
Citations
Citations to this article as recorded by- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
Sangmin Park, Yoonhyung Keum, Jaeyun Jeong, Seunghun Cha, Ju-Young Cho, Hyunchul Kim, Jiseong Lee, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Journal of Alloys and Compounds.2025; 1022: 178711. CrossRef - A Review of the Current Progress in High-Temperature Recycling Strategies for Recovery of Rare-Earth Elements from Magnet Waste
Ali Zakeri, Leili Tafaghodi
Journal of Sustainable Metallurgy.2025; 11(1): 88. CrossRef - Selective growth of Nb–Fe–B intermetallic compounds for the direct separation of rare earths based on manipulating liquation
Sangmin Park, Jaeyun Jeong, Seunghun Cha, Yoonhyung Keum, Ju-Young Cho, Hyungbeen Park, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Materials Today Sustainability.2024; 28: 101042. CrossRef - Separation and recovery Nd and Dy from Mg-REEs alloy by vacuum distillation
Sangmin Park, Dae-Kyeom Kim, Jaeyun Jeong, Jae Hong Shin, Yujin Kang, Rongyu Liu, Taek-Soo Kim, Myungsuk Song
Journal of Alloys and Compounds.2023; 967: 171775. CrossRef - The Supported Boro-Additive Effect for the Selective Recovery of Dy Elements from Rare-Earth-Elements-Based Magnets
Sangmin Park, Dae-Kyeom Kim, Javid Hussain, Myungsuk Song, Taek-Soo Kim
Materials.2022; 15(9): 3032. CrossRef - Influence of Dysprosium Compounds on the Extraction Behavior of Dy from Nd-Dy-Fe-B Magnet Using Liquid Magnesium
Sun-Woo Nam, Sang-Min Park, Mohammad Zarar Rasheed, Myung-Suk Song, Do-Hyang Kim, Taek-Soo Kim
Metals.2021; 11(9): 1345. CrossRef
- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
- [Korean]
- High Temperature Oxidation Behavior of 316L Austenitic Stainless Steel Manufactured by Laser Powder Bed Fusion Process
- Yu-Jin Hwang, Dong-Yeol Wi, Kyu-Sik Kim, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2021;28(2):110-119. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.110
- 1,185 View
- 26 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
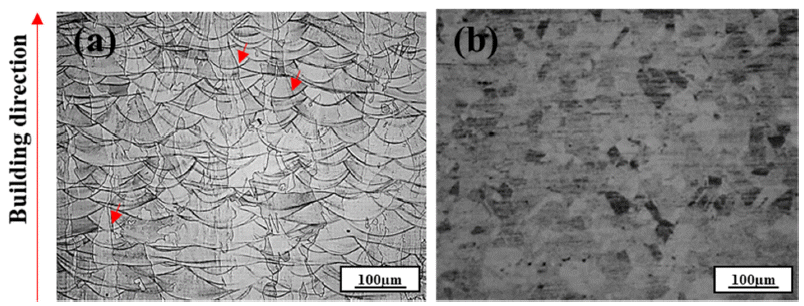
PDF In this study, the high-temperature oxidation properties of austenitic 316L stainless steel manufactured by laser powder bed fusion (LPBF) is investigated and compared with conventional 316L manufactured by hot rolling (HR). The initial microstructure of LPBF-SS316L exhibits a molten pool ~100 μm in size and grains grown along the building direction. Isotropic grains (~35 μm) are detected in the HR-SS316L. In high-temperature oxidation tests performed at 700°C and 900°C, LPBF-SS316L demonstrates slightly superior high-temperature oxidation resistance compared to HR-SS316L. After the initial oxidation at 700°C, shown as an increase in weight, almost no further oxidation is observed for both materials. At 900°C, the oxidation weight displays a parabolic trend and both materials exhibit similar behavior. However, at 1100°C, LPBF-SS316L oxidizes in a parabolic manner, but HR-SS316L shows a breakaway oxidation behavior. The oxide layers of LPBF-SS316L and HR-SS316L are mainly composed of Cr2O3, Febased oxides, and spinel phases. In LPBF-SS316L, a uniform Cr depletion region is observed, whereas a Cr depletion region appears at the grain boundary in HR-SS316L. It is evident from the results that the microstructure and the hightemperature oxidation characteristics and behavior are related.
-
Citations
Citations to this article as recorded by- Retention factor-based constitutive model of high-strength austenitic A4–80 bolts after fire exposure
Hui Wang, Bo Yang, Tao Sun, Weilai Yao, Wei Jiang
Journal of Constructional Steel Research.2025; 235: 109930. CrossRef - Study of structural stability at high temperature of pseudo-single tube with double layer as an alternative method for accident-tolerant fuel cladding
Jong Woo Kim, Hyeong Woo Min, Jaehwan Ko, Yonghee Kim, Young Soo Yoon
Journal of Nuclear Materials.2022; 566: 153800. CrossRef
- Retention factor-based constitutive model of high-strength austenitic A4–80 bolts after fire exposure
- [Korean]
- Evaluation of Oxygen Reduction and Surface Chemical State of Ti-48Al-2Cr-2Nb Powder by Ca Vapor
- Taeheon Kim, Hanjung Kwon, Jae-Won Lim
- J Korean Powder Metall Inst. 2021;28(1):31-37. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.31

- 1,094 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF This study explores reducing the oxygen content of a commercial Ti-48Al-2Cr-2Nb powder to less than 400 ppm by deoxidation in the solid state (DOSS) using Ca vapor, and investigates the effect of Ca vapor on the surface chemical state. As the deoxidation temperature increases, the oxygen concentration of the Ti-48Al-2Cr-2Nb powder decreases, achieving a low value of 745 ppm at 1100°C. When the deoxidation time is increased to 2 h, the oxygen concentration decreases to 320pp m at 1100°C, and the oxygen reduction rate is approximately 78% compared to that of the raw material. The deoxidized Ti-48Al-2Cr-2nb powder maintains a spherical shape, but the surface shape changes slightly owing to the reaction of Ca and Al. The oxidation state of Ti and Al on the surface of the Ti-48Al-2Cr-2Nb powder corresponds to a mixture of TiO2 and Al2O3. As a result, the peaks of metallic Ti and Ti suboxide intensify as TiO2 and Al2O3 in the surface oxide layer are reduced by Ca vapor deposition
-
Citations
Citations to this article as recorded by- Production of spherical TiAl alloy powder by copper-assisted spheroidization
Jin Qian, Bo Yin, Dashun Dong, Geng Wei, Ming Shi, Shaolong Tang
Journal of Materials Research and Technology.2023; 25: 1860. CrossRef - Ca-Mg Multiple Deoxidation of Ti-50Al-2Cr-2Nb Intermetallic Compound Powder for Additive Manufacturing
Seongjae Cho, Taeheon Kim, Jae-Won Lim
ECS Journal of Solid State Science and Technology.2022; 11(4): 045008. CrossRef
- Production of spherical TiAl alloy powder by copper-assisted spheroidization
- [Korean]
- Mechanical Properties and Thermal Stability of Ti0.5Al0.5N/CrN Nano-multilayered Coatings
- Seung-Su Ahn, Jong-Keuk Park, Kyung-Sik Oh, Tai-Joo Chung
- J Korean Powder Metall Inst. 2020;27(5):406-413. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.406

- 492 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Ti0.5Al0.5N/CrN nano-multilayers, which are known to exhibit excellent wear resistances, were prepared using the unbalanced magnetron sputter for various periods of 2–7 nm. Ti0.5Al0.5N and CrN comprised a cubic structure in a single layer with different lattice parameters; however, Ti0.5Al0.5N/CrN exhibited a cubic structure with the same lattice parameters that formed the superlattice in the nano-multilayers. The Ti0.5Al0.5/CrN multilayer with a period of 5.0 nm exceeded the hardness of the Ti0.5Al0.5N/CrN single layer, attaining a value of 36 GPa. According to the low-angle X-ray diffraction, the Ti0.5Al0.5N/CrN multilayer maintained its as-coated structure up to 700°C and exhibited a hardness of 32 GPa. The thickness of the oxidation layer of the Ti0.5Al0.5N/CrN multilayered coating was less than 25% of that of the single layers. Thus, the Ti0.5Al0.5N/CrN multilayered coating was superior in terms of hardness and oxidation resistance as compared to its constituent single layers.
- [English]
- Effect of SiC and WC additon on Oxidation Behavior of Spark-Plasma-Sintered ZrB2
- Chang-Yeoul Kim, Jae-Seok Choi, Sung-Churl Choi
- J Korean Powder Metall Inst. 2019;26(6):455-462. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.455
- 1,144 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF ZrB2 ceramic and ZrB2 ceramic composites with the addition of SiC, WC, and SiC/WC are successfully synthesized by a spark plasma sintering method. During high-temperature oxidation, SiC additive form a SiO2 amorphous outer scale layer and SiC-deplete ZrO2 scale layer, which decrease the oxidation rate. WC addition forms WO3 during the oxidation process to result in a ZrO2/WO3 liquid sintering layer, which is known to improve the antioxidation effect. The addition of SiC and WC to ZrB2 reduces the oxygen effective diffusivity by one-fifth of that of ZrB2. The addition of both SiC and WC shows the formation of a SiO2 outer dense glass layer and ZrO2/WO3 layer so that the anti-oxidation effect is improved three times as much as that of ZrB2. Therefore, SiC- and WC-added ZrB2 has a lower two-order oxygen effective diffusivity than ZrB2; it improves the anti-oxidation performance 3 times as much as that of ZrB2.
-
Citations
Citations to this article as recorded by- Role of TiC and WC Addition on the Mechanism and Kinetics of Isothermal Oxidation and High-Temperature Stability of ZrB2–SiC Composites
Pradyut Sengupta, Indranil Manna
High Temperature Corrosion of Materials.2024; 101(S1): 57. CrossRef
- Role of TiC and WC Addition on the Mechanism and Kinetics of Isothermal Oxidation and High-Temperature Stability of ZrB2–SiC Composites
- [Korean]
- Research Trends of the Mo-Si-B Alloys as Next Generation Ultra-high-temperature Alloys
- Won June Choi, Chun Woong Park, Jung Hyo Park, Young Do Kim, Jong Min Byun
- J Korean Powder Metall Inst. 2019;26(2):156-165. Published online April 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.2.156

- 1,492 View
- 26 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF Over the last decade, the next generation’s ultra-high-temperature materials as an alternative to Nickel-based superalloys have been highlighted. Ultra-high-temperature materials based on refractory metals are one of several potential candidates. In particular, molybdenum alloys with small amounts of silicon and boron (Mo-Si-B alloys) have superior properties at high temperature. However, research related to Mo-Si-B alloys were mainly conducted by several developed countries but garnered little interest in Korea. Therefore, in this review paper, we introduce the development history of Mo-Si-B alloys briefly and discuss the properties, particularly the mechanical and oxidation properties of Mo-Si-B alloys. We also introduce the latest research trends of Mo-Si-B alloys based on the research paper. Finally, for domestic research related to this field, we explain why Mo-Si-B alloys should be developed and suggest the potential directions for Mo-Si-B alloys research.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef - Preparation and Structure of Chromium Coatings Doped with Diamond Nanoparticles Deposited Directly on a Monolithic Composite of Molybdenum and Aluminum
V. P. Petkov, M. K. Aleksandrova, R. V. Valov, V. P. Korzhov, V. M. Kiiko, I. S. Zheltyakova
Protection of Metals and Physical Chemistry of Surfaces.2023; 59(3): 396. CrossRef - A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials
Liang Jiang, Bin Zheng, Changsong Wu, Pengxiang Li, Tong Xue, Jiandong Wu, Fenglan Han, Yuhong Chen
Processes.2022; 10(9): 1772. CrossRef - Heat-Resistant Molybdenum Borosilicate Alloys Hardened with Titanium Carbides: Mo–Si–B–TiC (Survey)
I. L. Svetlov, O. G. Ospennikova, M. I. Karpov, Yu. V. Artemenko
Inorganic Materials: Applied Research.2021; 12(4): 866. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
- Lattice Deformation and Improvement Oxidation Resistance of Ti-6Al-4V Alloy Powders Prepared by Hydrogen Added Argon Heat Treatment
- Gye-Hoon Cho, Jung-Min Oh, Jae-Won Lim
- J Korean Powder Metall Inst. 2019;26(2):126-131. Published online April 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.2.126

- 545 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF In the present work, a new hydrogen added argon heat treatment process that prevents the formation of hydrides and eliminates the dehydrogenation step, is developed. Dissolved hydrogen has a good effect on sintering properties such as oxidation resistance and density of greens. This process can also reduce costs and processing time. In the experiment, commercially available Ti-6Al-4V powders are used. The powders are annealed using tube furnace in an argon atmosphere at 700°C and 900°C for 120 min. Hydrogen was injected temporarily during argon annealing to dissolve hydrogen, and a dehydrogenation process was performed simultaneously under an argon-only atmosphere. Without hydride formation, hydrogen was dissolved in the Ti-6Al-4V powder by X-ray diffraction and gas analysis. Hydrogen is first solubilized on the beta phase and expanded the beta phases’ cell volume. TGA analysis was carried out to evaluate the oxidation resistance, and it is confirmed that hydrogen-dissolved Ti-6Al-4V powders improves oxidation resistance more than raw materials.
- [Korean]
- Synthesis of TiO2 Nanowires by Thermal Oxidation of Titanium Alloy Powder
- Yoo-Young Kim, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2018;25(1):48-53. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.48

- 735 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF One-dimensional rutile TiO2 is an important inorganic compound with applicability in sensors, solar cells, and Li-based batteries. However, conventional synthesis methods for TiO2 nanowires are complicated and entail risks of environmental contamination. In this work, we report the growth of TiO2 nanowires on a Ti alloy powder (Ti-6wt%Al-4wt%V, Ti64) using simple thermal oxidation under a limited supply of O2. The optimum condition for TiO2 nanowire synthesis is studied for variables including temperature, time, and pressure. TiO2 nanowires of ~5 μm in length and 100 nm in thickness are richly synthesized under the optimum condition with single-crystalline rutile phases. The formation of TiO2 nanowires is greatly influenced by synthesis temperature and pressure. The synthesized TiO2 nanowires are characterized using field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and high-resolution transmission electron microscopy (HR-TEM).
- [Korean]
- Formation of Uniform SnO2 Coating Layer on Carbon Nanofiber by Pretreatment in Atomic Layer Deposition
- Dong Ha Kim, Doh-Hyung Riu, Byung Joon Choi
- J Korean Powder Metall Inst. 2018;25(1):43-47. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.43
- 815 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
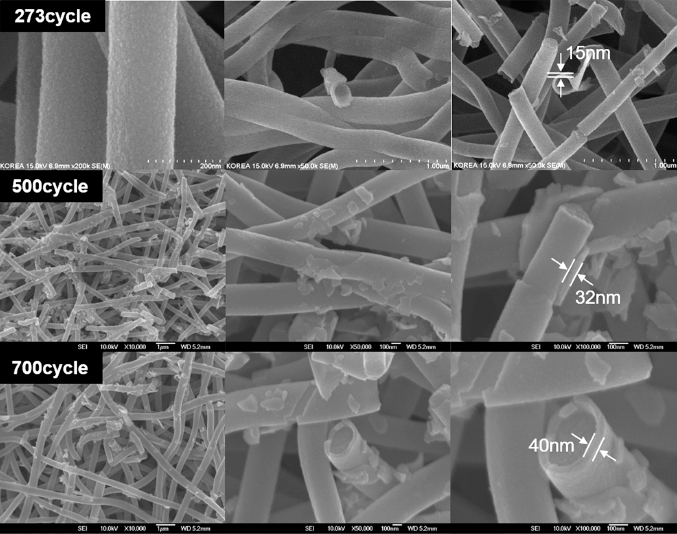
PDF Carbon nanofibers (CNF) are widely used as active agents for electrodes in Li-ion secondary battery cells, supercapacitors, and fuel cells. Nanoscale coatings on CNF electrodes can increase the output and lifespan of battery devices. Atomic layer deposition (ALD) can control the coating thickness at the nanoscale regardless of the shape, suitable for coating CNFs. However, because the CNF surface comprises stable C–C bonds, initiating homogeneous nuclear formation is difficult because of the lack of initial nucleation sites. This study introduces uniform nucleation site formation on CNF surfaces to promote a uniform SnO2 layer. We pretreat the CNF surface by introducing H2O or Al2O3 (trimethylaluminum + H2O) before the SnO2 ALD process to form active sites on the CNF surface. Transmission electron microscopy and energy-dispersive spectroscopy both identify the SnO2 layer morphology on the CNF. The Al2O3-pretreated sample shows a uniform SnO2 layer, while island-type SnOx layers grow sparsely on the H2Opretreated or untreated CNF.
-
Citations
Citations to this article as recorded by- Atomic layer deposition of ZnO layers on Bi2Te3 powders: Comparison of gas fluidization and rotary reactors
Myeong Jun Jung, Myeongjun Ji, Jeong Hwan Han, Young-In Lee, Sung-Tag Oh, Min Hwan Lee, Byung Joon Choi
Ceramics International.2022; 48(24): 36773. CrossRef - Effects of SnO2 layer coated on carbon nanofiber for the methanol oxidation reaction
Dong Ha Kim, Dong-Yo Shin, Young-Geun Lee, Geon-Hyoung An, Jeong Hwan Han, Hyo-Jin Ahn, Byung Joon Choi
Ceramics International.2018; 44(16): 19554. CrossRef
- Atomic layer deposition of ZnO layers on Bi2Te3 powders: Comparison of gas fluidization and rotary reactors
- [Korean]
- The Effect of SiO2 addition on Oxidation and Electrical Resistance Stability at High-temperature of P/M Fecralloy Compact
- Jin-Woo Park, Jin-Uk Ok, Woo-young Jung, Dong-kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2017;24(4):292-297. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.292
- 638 View
- 2 Download
-
 Abstract
Abstract
 PDF
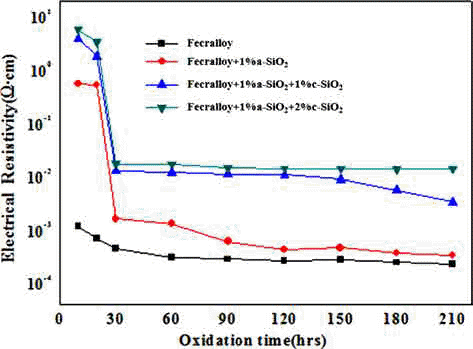
PDF A metallic oxide layer of a heat-resistant element contributes to the high-temperature oxidation resistance by delaying the oxidation and has a positive effect on the increase in electrical resistivity. In this study, green compacts of Fecralloy powder mixed with amorphous and crystalline silica are oxidized at 950°C for up to 210 h in order to evaluate the effect of metal oxide on the oxidation and electrical resistivity. The weight change ratio increases as per a parabolic law, and the increase is larger than that observed for Fecralloy owing to the formation of Fe-Si, Fe-Cr composite oxide, and Al2O3 upon the addition of Si oxide. Si oxides promote the formation of Al2O3 and Cr oxide at the grain boundary, and obstruct neck formation and the growth of Fecralloy particles to ensure stable electrical resistivity.
- [Korean]
- High Temperature Oxidation Behavior of Fe-14Cr Ferritic Oxide Dispersion Strengthened Steels Manufactured by Mechanical Alloying Process
- Young-Kyun Kim, Jong-Kwan Park, Hwi-Jun Kim, Man-Sik Kong, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2017;24(2):133-140. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.133
- 679 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
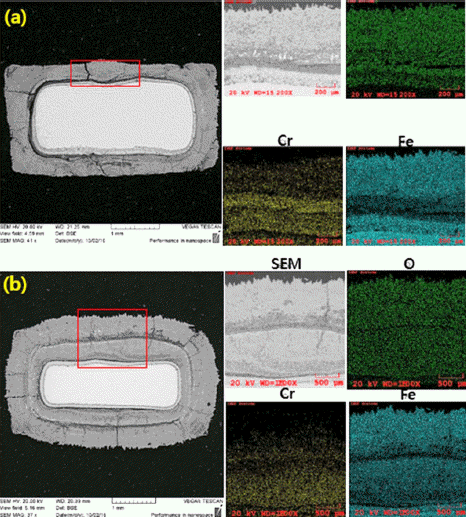
PDF This study investigates the oxidation properties of Fe-14Cr ferritic oxide-dispersion-strengthened (ODS) steel at various high temperatures (900, 1000, and 1100°C for 24 h). The initial microstructure shows that no clear structural change occurs even under high-temperature heat treatment, and the average measured grain size is 0.4 and 1.1 μm for the as-fabricated and heat-treated specimens, respectively. Y–Ti–O nanoclusters 10–50 nm in size are observed. High-temperature oxidation results show that the weight increases by 0.27 and 0.29 mg/cm2 for the asfabricated and heat-treated (900°C) specimens, and by 0.47 and 0.50 mg/cm2 for the as-fabricated and heat-treated (1000°C) specimens, respectively. Further, after 24 h oxidation tests, the weight increases by 56.50 and 100.60 mg/cm2 for the as-fabricated and heat-treated (1100°C) specimens, respectively; the latter increase is approximately 100 times higher than that at 1000°C. Observation of the surface after the oxidation test shows that Cr2O3 is the main oxide on a specimen tested at 1000°C, whereas Fe2O3 and Fe3O4 phases also form on a specimen tested at 1100°C, where the weight increases rapidly. The high-temperature oxidation behavior of Fe-14Cr ODS steel is confirmed to be dominated by changes in the Cr2O3 layer and generation of Fe-based oxides through evaporation.
-
Citations
Citations to this article as recorded by- Microstructure and Wear Properties of Oxide Dispersion Strengthened Steel Powder Added Steel-Based Composite Material for Automotive Part
Young-Kyun Kim, Jong-Kwan Park, Kee-Ahn Lee
journal of Korean Powder Metallurgy Institute.2018; 25(1): 36. CrossRef
- Microstructure and Wear Properties of Oxide Dispersion Strengthened Steel Powder Added Steel-Based Composite Material for Automotive Part
- [Korean]
- Microstructure and High Temperature Oxidation Behaviors of Fe-Ni Alloys by Spark Plasma Sintering
- Chae Hong Lim, Jong Seok Park, Sangsun Yang, Jung-Yeul Yun, Jin Kyu Lee
- J Korean Powder Metall Inst. 2017;24(1):53-57. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.53

- 515 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF In this study, we report the microstructure and the high-temperature oxidation behavior of Fe-Ni alloys by spark plasma sintering. Structural characterization is performed by scanning electron microscopy and X-ray diffraction. The oxidation behavior of Fe-Ni alloys is studied by means of a high-temperature oxidation test at 1000°C in air. The effect of Ni content of Fe-Ni alloys on the microstructure and on the oxidation characteristics is investigated in detail. In the case of Fe-2Ni and Fe-5Ni alloys, the microstructure is a ferrite (α) phase with body centered cubic (BCC) structure, and the microstructure of Fe-10Ni and Fe-20Ni alloys is considered to be a massive martensite (α’) phase with the same BCC structure as that of the ferrite phase. As the Ni content increases, the micro-Vickers hardness of the alloys also increases. It can also be seen that the oxidation resistance is improved by decreasing the thickness of the oxide film.
- [Korean]
- Basic Study on the Recycling of Waste Tungsten Scraps by the Oxidation and Reduction Process
- Sang-Uk Kim, Ji-seok Yun, Tae-Wook Kim, Bong-Hwi Cho, In-Ho Kim, Sang-Mu Kim, Chang-Bin Song
- J Korean Powder Metall Inst. 2017;24(1):34-40. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.34

- 784 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF This study is carried out to obtain basic data regarding oxidation and reduction reactions, originated on the recycling of waste tungsten hard scraps by oxidation and reduction processes. First, it is estimated that the theoretical Gibbs free energy for the formation reaction of WO2 and WO3 are calculated as ΔG1,000K= -407.335 kJ/mol and ΔG1,000K = -585.679 kJ/mol, from the thermodynamics data reported by Ihsan Barin. In the experiments, the oxidation of pure tungsten rod by oxygen is carried out over a temperature range of 700-1,000°C for 1 h, and it is possible to conclude that the oxidation reaction can be represented by a relatively linear relationship. Second, the reduction of WO2 and WO3 powder by hydrogen is also calculated from the same thermodynamics data, and it can be found that it was difficult for the reduction reaction to occur at 1,027°C, in the case of WO2, but it can happen for temperatures higher than 1127°C. On the other hand, WO3 reduction reaction occurs at the relatively low temperature of 827oC. Based on these results, the reduction experiments are carried out at a temperature range of 500-1,000°C for 15 min to 4 h, in the case of WO3 powder, and it is possible to conclude that the reduction at 900°C for 2h is needed for a perfect reduction reaction.
-
Citations
Citations to this article as recorded by- A Basic Study on the Recycling of Wasted Cemented Carbide by the Zn Bath Process(Ⅰ)
Kyung-Sik Kim, In-Ho Kim, Chan-Gi Lee, Chang-Bin Song
Journal of the Korean Institute of Resources Recycling.2020; 29(6): 35. CrossRef
- A Basic Study on the Recycling of Wasted Cemented Carbide by the Zn Bath Process(Ⅰ)
- [Korean]
- A Study on the Development of Compactability and Electrical Resistivity for P/M Fecralloy
- Jin-Woo Parka, Byung-Hyun Ko, Woo-Young Jung, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2016;23(6):426-431. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.426
- 589 View
- 2 Download
-
 Abstract
Abstract
 PDF
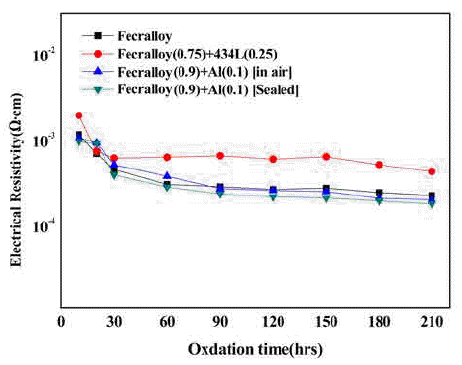
PDF The Fe-Cr-Al alloy system shows an excellent heat resistance because of the formation of an Al2O3 film on the metal surface in an oxidizing atmosphere at high temperatures up to 1400°C. The Fecralloy needs an additive that can act as a binder because of its bad compactability. In this study, the green compacts of STS434L and Al powder added to Fecralloy are oxidized at 950°C for up to 210 h. Fecralloy and Al is mixed by two types of ball milling. One is vented to air and the other was performed in a sealed jar. In the case of Al addition, there are no significant changes in the electrical resistance. Before the oxidation test, Al oxides are present in the Fecralloy surface, as determined from the energy dispersive spectroscopy results. The addition of Al improves the compactability because of an increased density, and the addition of STS434L increases the electrical resistivity by forming a composite oxide.
- [Korean]
- The Effect of Oxide Compound on Electrical Resistivity and Oxidation Stability in High-temperature for Ferritic P/M Stainless Steel
- Jin-Woo Park, Byung-Hyun Ko, Woo-young Jung, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2016;23(3):240-246. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.240

- 597 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF In order to improve the high-temperature oxidation stability, sintered 434L stainless steel is studied, focusing on the effect of the addition of metallic oxides to form stable oxide films on the inner particle surface. The green compacts of Fecralloy powder or amorphous silica are added on STS434L and oxidized at 950°C up to 210 h. The weight change ratio of 434L with amorphous silica is higher than that of 434L mixed with Fecralloy, and the weight increase follows a parabolic law, which implies that the oxide film grows according to oxide diffusion through the densely formed oxide film. In the case of 434L mixed with Fecralloy, the elements in the matrix diffuse through the grain boundaries and form Al2O3 and Fe-Cr oxides. Stable high temperature corrosion resistance and electrical resistivity are obtained for STS434L mixed with Fecralloy.
- [Korean]
- The Effect of Oxides Additives on Anti-corrosion Properties of Sintered 316L Stainless Steel
- Jong-Pil Lee, Ji-Hyun Hong, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2015;22(4):271-277. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.271

- 597 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF As wrought stainless steel, sintered stainless steel (STS) has excellent high-temperature anti-corrosion even at high temperature of 800ºC and exhibit corrosion resistance in air. The oxidation behavior and oxidation mechanism of the sintered 316L stainless was reported at the high temperature in our previous study. In this study, the effects of additives on high-temperature corrosion resistances were investigated above 800ºC at the various oxides (SiO2, Al2O3, MgO and Y2O3) added STS respectively as an oxidation inhibitor. The morphology of the oxide layers were observed by SEM and the oxides phase and composition were confirmed by XRD and EDX. As a result, the weight of STS 316L sintered body increased sharply at 1000oC and the relative density of specimen decreased as metallic oxide addition increased. Compared with STS 316L sintered parts, weight change ratio corresponding to different oxidation time at 900oC and 1000oC, decreased gradually with the addition of metallic oxide. The best corrosion resistance properties of STS could be improved in case of using Y2O3. The oxidation rate was diminished dramatically by suppression the peeling on oxide layers at Y2O3 added sintered stainless steel.
- [Korean]
- The Effects of Composition and Microstructure Variation on the Oxidation Characteristics of Stainless Steels Manufactured by Powder Metallurgy Method
- Jong-Pil Lee, Ji-Hyun Hong, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2015;22(1):52-59. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.52

- 1,297 View
- 11 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF As well-known wrought stainless steel, sintered stainless steel (STS) has excellent high-temperature anticorrosion even at high temperature of 800°C, and exhibits good corrosion resistance in air. However, when temperature increases above 900°C, the corrosion resistance of STS begins to deteriorate and dramatically decreases. In this study, the effects of phase and composition of STS on high-temperature corrosion resistances are investigated for STS 316L, STS 304 and STS 434L above 800°C. The morphology of the oxide layers are observed. The oxides phase and composition are identified using X-ray diffractometer and energy dispersive spectroscopy. The results demonstrate that the best corrosion resistance of STS could be improved to that of 434L. The poor corrosion resistance of the austenitic stainless steels is due to the fact that NiFe2O4 oxides forming poor adhesion between the matrix and oxide film increase the oxidation susceptibility of the material at high temperature.
-
Citations
Citations to this article as recorded by- The effect of different turbulent flow on failure behavior in secondary loop of the pressurized water reactor
Y. Hu, L. Zhao, Y.H. Lu, T. Shoji
Nuclear Engineering and Design.2020; 368: 110812. CrossRef
- The effect of different turbulent flow on failure behavior in secondary loop of the pressurized water reactor
- [Korean]
- Effect of Cell Size on the High Temperature Oxidation Properties of Fe-Cr-Al Powder Porous Metal Manufactured by Electro-spray Process
- Jae-Sung Oh, Young-Min Kong, Byoung-Kee Kim, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2014;21(1):55-61. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.55

- 978 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Fe-Cr-Al powder porous metal was manufactured by using new electro-spray process. First, ultra-fine fecralloy powders were produced by using the submerged electric wire explosion process. Evenly distributed colloid (0.05~0.5% powders) was dispersed on Polyurethane foam through the electro-spray process. And then degreasing and sintering processes were conduced. In order to examine the effect of cell size (200 μm, 450 μm, 500 μm) in process, pre-samples were sintered for two hours at temperature of 1450°C, in H2 atmospheres. A 24-hour thermo gravimetric analysis test was conducted at 1000°C in a 79% N2 + 21% O2 to investigate the high temperature oxidation behavior of powder porous metal. The results of the high temperature oxidation tests showed that oxidation resistance increased with increasing cell size. In the 200 μm porous metal with a thinner strut and larger specific surface area, the depletion of the stabilizing elements such as Al and Cr occurred more quickly during the high-temperature oxidation compared with the 450, 500 μm porous metals.
-
Citations
Citations to this article as recorded by- Fabrication and Mechanical Properties of Open‐Cell Austenitic Stainless Steel Foam by Electrostatic Powder Spraying Process
Tae-Hoon Kang, Kyu-Sik Kim, Jung-Yeul Yun, Min-Jeong Lee, Kee-Ahn Lee
Advanced Engineering Materials.2020;[Epub] CrossRef - Microstructure and High Temperature Oxidation Behaviors of Fe-Ni Alloys by Spark Plasma Sintering
Chae Hong Lim, Jong Seok Park, Sangsun Yang, Jung-Yeul Yun, Jin Kyu Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(1): 53. CrossRef - Effect of Al2O3 Inter-Layer Grown on FeCrAl Alloy Foam to Improve the Dispersion and Stability of NiO Catalysts
유진 이, 본율 구, 성호 백, 만호 박, 효진 안
Korean Journal of Materials Research.2015; 25(8): 391~397. CrossRef
- Fabrication and Mechanical Properties of Open‐Cell Austenitic Stainless Steel Foam by Electrostatic Powder Spraying Process
TOP
 KPMI
KPMI


 First
First Prev
Prev


