Search
- Page Path
- HOME > Search
- [Korean]
- Comparison of the Properties of Rare-Earth Zirconate Thermal Barrier Coatings for Hydrogen-Fueled Gas Turbines
- Gun-Woong Lee, Min-Soo Nam, Min-Ji Kim, HyunSuk Jung, Seongwon Kim
- J Powder Mater. 2025;32(6):472-480. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00423

- 734 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF - Thermal barrier coatings (TBCs) for hydrogen-fueled gas turbines withstand higher combustion temperatures and increased steam concentrations compared to conventional natural-gas systems. These harsh operating conditions significantly accelerate the thermal degradation of widely used YSZ coatings, emphasizing the need for alternative top-coat materials with improved phase stability and reduced thermal conductivity. In this study, rare-earth zirconate ceramics, Gd2Zr2O7 (GdZO), Tm2Zr2O7 (TmZO), and a mixed composition (Gd0.5Tm0.5)2Zr2O7 (Gd/TmZO), are synthesized and investigated as potential next-generation TBC candidates. Each material was comparatively examined with a focus on crystal structure, thermophysical properties, and thermal conductivity. Furthermore, high-temperature steam exposure experiments were performed to simulate hydrogen combustion environments. Microstructural analyses, high-temperature degradation behavior, and phase stability evaluations were carried out to obtain fundamental experimental data. This study provides essential baseline information for the design and development of high-performance TBC materials suitable for the hydrogen-fueled gas turbine systems.
- [Korean]
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(4):324-328. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00199

- 1,146 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - This study analyzed the influence of ball size and process control agents on the refinement and dehydrogenation behavior of TiH2 powder. Powders milled using ZrO2 balls with diameters of 0.1 mm, 0.3 mm, and 0.3+0.5+1 mm exhibited a bimodal particle size distribution, of which the first mode had the smallest size of 0.23 μm for the 0.3 mm balls. Using ethanol and/or stearic acid as process control agents was effective in particle refinement. Thermogravimetric analysis showed that dehydrogenation of the milled powder started at a relatively low temperature compared to the raw powder, which is interpreted to have resulted from a decrease in particle size and an increase in defects. The dehydrogenation kinetics of the TiH2 powder were evaluated by the magnitude of peak shift with heating rates using thermogravimetric analysis. The activation energy of the dehydrogenation reaction, calculated from the slope of the Kissinger plot, was measured to be 228.6 kJ/mol for the raw powder and 194.5 kJ/mol for the milled powder. TEM analysis revealed that both the milled and dehydrogenated powders showed an angular shape with a size of about 200 nm.
- [Korean]
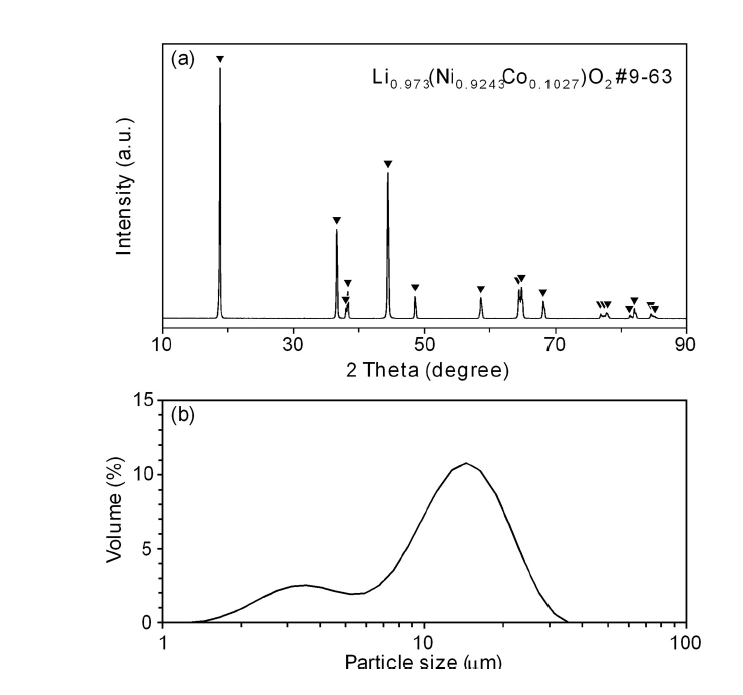
- Hydrogen Reduction Behavior of NCM-based Lithium-ion Battery Cathode Materials
- So-Yeong Lee, So-Yeon Lee, Dae-Hyeon Lee, Ho-Sang Sohn
- J Powder Mater. 2024;31(2):163-168. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00017
- 1,650 View
- 42 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - As the demand for lithium-ion batteries for electric vehicles is increasing, it is important to recover valuable metals from waste lithium-ion batteries. In this study, the effects of gas flow rate and hydrogen partial pressure on hydrogen reduction of NCM-based lithium-ion battery cathode materials were investigated. As the gas flow rate and hydrogen partial pressure increased, the weight loss rate increased significantly from the beginning of the reaction due to the reduction of NiO and CoO by hydrogen. At 700 °C and hydrogen partial pressure above 0.5 atm, Ni and Li2O were produced by hydrogen reduction. From the reduction product and Li recovery rate, the hydrogen reduction of NCM-based cathode materials was significantly affected by hydrogen partial pressure. The Li compounds recovered from the solution after water leaching of the reduction products were LiOH, LiOH·H2O, and Li2CO3, with about 0.02 wt% Al as an impurity.
-
Citations
Citations to this article as recorded by- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef
- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
- [Korean]
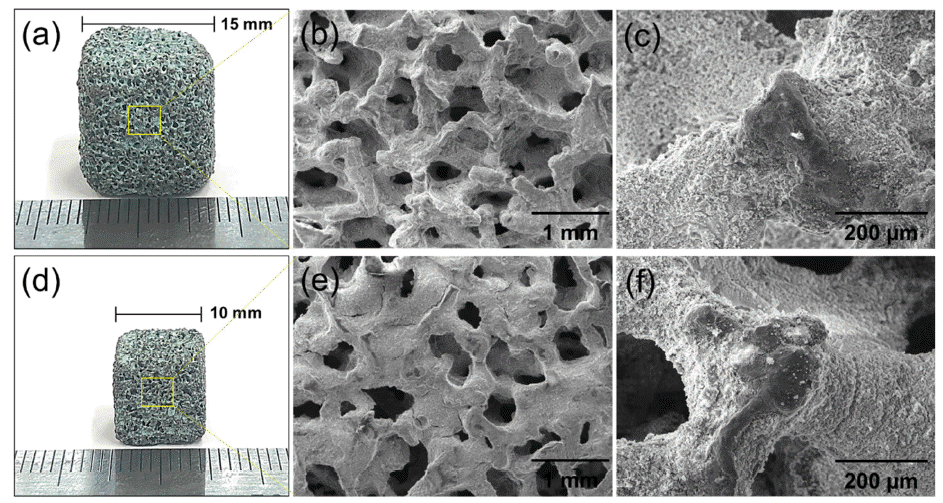
- Fabrication of Ni-Cr-Al Metal Foam-Supported Catalysts for the Steam Methane Reforming (SMR), and its Mechanical Stability and Hydrogen Yield Efficiency
- Kyu-Sik Kim, Tae-Hoon Kang, Man Sik Kong, Man-Ho Park, Jung-Yeul Yun, Ji Hye Ahn, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2021;28(3):201-207. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.201
- 1,284 View
- 19 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Ni–Cr–Al metal-foam-supported catalysts for steam methane reforming (SMR) are manufactured by applying a catalytic Ni/Al2O3 sol–gel coating to powder alloyed metallic foam. The structure, microstructure, mechanical stability, and hydrogen yield efficiency of the obtained catalysts are evaluated. The structural and microstructural characteristics show that the catalyst is well coated on the open-pore Ni–Cr–Al foam without cracks or spallation. The measured compressive yield strengths are 2–3 MPa at room temperature and 1.5–2.2 MPa at 750°C regardless of sample size. The specimens exhibit a weight loss of up to 9–10% at elevated temperature owing to the spallation of the Ni/Al2O3 catalyst. However, the metal-foam-supported catalyst appears to have higher mechanical stability than ceramic pellet catalysts. In SMR simulations tests, a methane conversion ratio of up to 96% is obtained with a high hydrogen yield efficiency of 82%.
-
Citations
Citations to this article as recorded by- The Experimental Investigation of a 98% Hydrogen Peroxide Monopropellant Thruster Comprising the Metal-Foam-Supported Manganese Oxide Catalyst
Pawel Surmacz, Zbigniew Gut
Aerospace.2023; 10(3): 215. CrossRef
- The Experimental Investigation of a 98% Hydrogen Peroxide Monopropellant Thruster Comprising the Metal-Foam-Supported Manganese Oxide Catalyst
- [Korean]
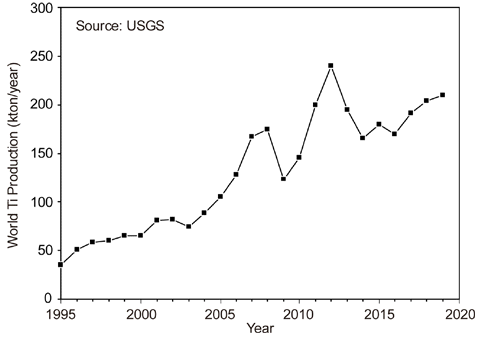
- Current Status of Titanium Smelting Technology for Powder Metallurgy
- Ho-Sang Sohn
- J Korean Powder Metall Inst. 2021;28(2):164-172. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.164
- 810 View
- 8 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Titanium is the ninth most abundant element in the Earth’s crust and is the fourth most abundant structural metal after aluminum, iron, and magnesium. It exhibits a higher specific strength than steel along with an excellent corrosion resistance, highlighting the promising potential of titanium as a structural metal. However, titanium is difficult to extract from its ore and is classified as a rare metal, despite its abundance. Therefore, the production of titanium is exceedingly low compared to that of common metals. Titanium is conventionally produced as a sponge by the Kroll process. For powder metallurgy (PM), hydrogenation-dehydrogenation (HDH) of the titanium sponge or gas atomization of the titanium bulk is required. Therefore, numerous studies have been conducted on smelting, which replaces the Kroll process and produces powder that can be used directly for PM. In this review, the Kroll process and new smelting technologies of titanium for PM, such as metallothermic, electrolytic, and hydrogen reduction of TiCl4 and TiO2 are discussed.
-
Citations
Citations to this article as recorded by- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
Yeon Joo Lee, Hyokyung Sung, Jae Bok Seol, Kisub Cho, Hwi Jun Kim, Hyunjoo Choi
Powder Metallurgy.2025; 68(3): 230. CrossRef
- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
- [Korean]
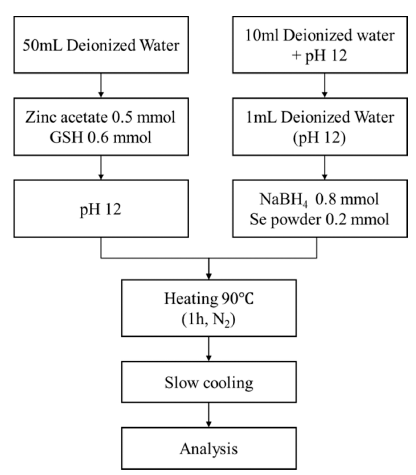
- Effects of Synthesis Conditions on Luminescence Characteristics of Glutathione Capped ZnSe Nano particles
- Geum Ji Back, Ha Yeon Song, Min Seo Lee, Hyun Seon Hong
- J Korean Powder Metall Inst. 2021;28(1):44-50. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.44
- 1,195 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Zinc selenide (ZnSe) nanoparticles were synthesized in aqueous solution using glutathione (GSH) as a ligand. The influence of the ligand content, reaction temperature, and hydroxyl ion concentration (pH) on the fabrication of the ZnSe particles was investigated. The optical properties of the synthesized ZnSe particles were characterized using various analytical techniques. The nanoparticles absorbed UV-vis light in the range of 350-400 nm, which is shorter than the absorption wavelength of bulk ZnSe particles (460 nm). The lowest ligand concentration for achieving good light absorption and emission properties was 0.6 mmol. The reaction temperature had an impact on the emission properties; photoluminescence spectroscopic analysis showed that the photo-discharge characteristics were greatly enhanced at high temperatures. These discharge characteristics were also affected by the hydroxyl ion concentration in solution; at pH 13, sound emission characteristics were observed, even at a low temperature of 25°C. The manufactured nanoparticles showed excellent light absorption and emission properties, suggesting the possibility of fabricating ZnSe QDs in aqueous solutions at low temperatures.
-
Citations
Citations to this article as recorded by- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
Geum Ji Back, Ha Yeon Song, Min Seo Lee, Jaesik Yoon, Hyun Seon Hong
Journal of Crystal Growth.2024; 626: 127475. CrossRef - Effect of UV Irradiation on Optical Properties of Water-Based Synthetic Zinc Selenide Quantum Dots
Geum Ji Back, Yu Jin Kang, I Ju Kang, Jeong Hyeon Lim, Hyun Seon Hong
Korean Journal of Metals and Materials.2022; 60(2): 160. CrossRef
- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
- [Korean]
- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
- Gyuhwi Lee, Ju-Yeon Han, Sung-Tag Oh
- J Korean Powder Metall Inst. 2020;27(3):193-197. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.193
- 721 View
- 3 Download
-
 Abstract
Abstract
 PDF
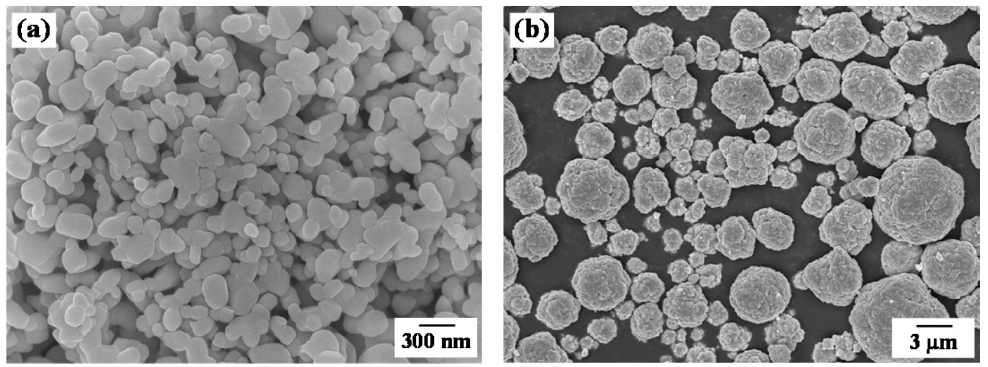
PDF Porous Cu-14 wt% Co with aligned pores is produced by a freeze drying and sintering process. Unidirectional freezing of camphene slurry with CuO-Co3O4 powders is conducted, and pores in the frozen specimens are generated by sublimation of the camphene crystals. The dried bodies are hydrogen-reduced at 500°C and sintered at 800°C for 1 h. The reduction behavior of the CuO-Co3O4 powder mixture is analyzed using a temperature-programmed reduction method in an Ar-10% H2 atmosphere. The sintered bodies show large and aligned parallel pores in the camphene growth direction. In addition, small pores are distributed around the internal walls of the large pores. The size and fraction of the pores decrease as the amount of solid powder added to the slurry increases. The change in pore characteristics according to the amount of the mixed powder is interpreted to be due to the rearrangement and accumulation behavior of the solid particles in the freezing process of the slurry.
- [Korean]
- Hydrogen Reduction Behavior and Microstructure Characteristics of Ball-milled CuO-Co3O4 Powder Mixtures
- Ju-Yeon Han, Gyuhwi Lee, Hyunji Kang, Sung-Tag Oh
- J Korean Powder Metall Inst. 2019;26(5):410-414. Published online October 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.5.410
- 740 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The hydrogen reduction behavior of the CuO-Co3O4 powder mixture for the synthesis of the homogeneous Cu-15at%Co composite powder has been investigated. The composite powder is prepared by ball milling the oxide powders, followed by a hydrogen reduction process. The reduction behavior of the ball-milled powder mixture is analyzed by X-ray diffraction (XRD) and temperature-programmed reduction at different heating rates in an Ar-10%H2 atmosphere. The scanning electron microscopy and XRD results reveal that the hydrogen-reduced powder mixture is composed of fine agglomerates of nanosized Cu and Co particles. The hydrogen reduction kinetics is studied by determining the degree of peak shift as a function of the heating rate. The activation energies for the reduction of the oxide powders estimated from the slopes of the Kissinger plots are 58.1 kJ/mol and 65.8 kJ/mol, depending on the reduction reaction: CuO to Cu and Co3O4 to Co, respectively. The measured temperature and activation energy for the reduction of Co3O4 are explained on the basis of the effect of pre-reduced Cu particles.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
Gyuhwi Lee, Ju-Yeon Han, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2020; 27(3): 193. CrossRef
- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
- [Korean]
- The Microstructure and the Mechanical Properties of Sintered TiO2-Co Composite Prepared Via Thermal Hydrogenation Method
- Myeongsun Ko, Ilsong Park, Jeshin Park
- J Korean Powder Metall Inst. 2019;26(4):290-298. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.290
- 389 View
- 1 Download
-
 Abstract
Abstract
 PDF
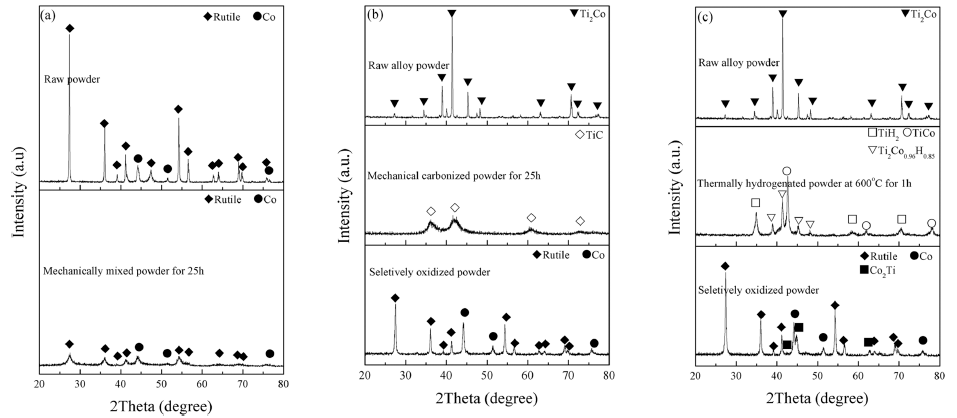
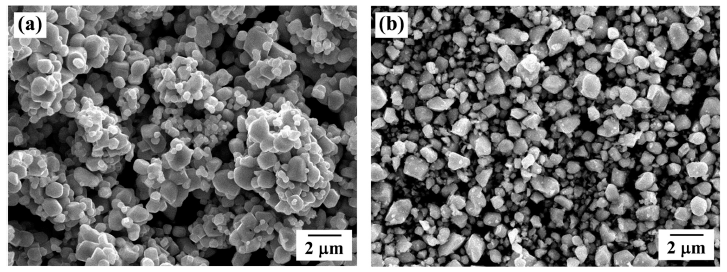
PDF TiO2-particles containing Co grains are fabricated via thermal hydrogenation and selective oxidation of Ti-Co alloy. For comparison, TiO2-Co composite powders are prepared by two kinds of methods which were the mechanical carbonization and oxidation process, and the conventional mixing process. The microstructural characteristics of the prepared composites are analyzed by X-ray diffraction, field-emission scattering electron microscopy, and transmission electron microscopy. In addition, the composite powders are sintered at 800°C by spark plasma sintering. The flexural strength and fracture toughness of the sintered samples prepared by thermal hydrogenation and mechanical carbonization are found to be higher than those of the samples prepared by the conventional mixing process. Moreover, the microstructures of sintered samples prepared by thermal hydrogenation and mechanical carbonization processes are found to be similar. The difference in the mechanical properties of sintered samples prepared by thermal hydrogenation and mechanical carbonization processes is attributed to the different sizes of metallic Co particles in the samples.
- [Korean]
- Lattice Deformation and Improvement Oxidation Resistance of Ti-6Al-4V Alloy Powders Prepared by Hydrogen Added Argon Heat Treatment
- Gye-Hoon Cho, Jung-Min Oh, Jae-Won Lim
- J Korean Powder Metall Inst. 2019;26(2):126-131. Published online April 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.2.126

- 545 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF In the present work, a new hydrogen added argon heat treatment process that prevents the formation of hydrides and eliminates the dehydrogenation step, is developed. Dissolved hydrogen has a good effect on sintering properties such as oxidation resistance and density of greens. This process can also reduce costs and processing time. In the experiment, commercially available Ti-6Al-4V powders are used. The powders are annealed using tube furnace in an argon atmosphere at 700°C and 900°C for 120 min. Hydrogen was injected temporarily during argon annealing to dissolve hydrogen, and a dehydrogenation process was performed simultaneously under an argon-only atmosphere. Without hydride formation, hydrogen was dissolved in the Ti-6Al-4V powder by X-ray diffraction and gas analysis. Hydrogen is first solubilized on the beta phase and expanded the beta phases’ cell volume. TGA analysis was carried out to evaluate the oxidation resistance, and it is confirmed that hydrogen-dissolved Ti-6Al-4V powders improves oxidation resistance more than raw materials.
- [Korean]
- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
- Jae-Hun Jeong, Sung-Tag Oh, Chang-Yong Hyun
- J Korean Powder Metall Inst. 2019;26(1):6-10. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.6
- 913 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
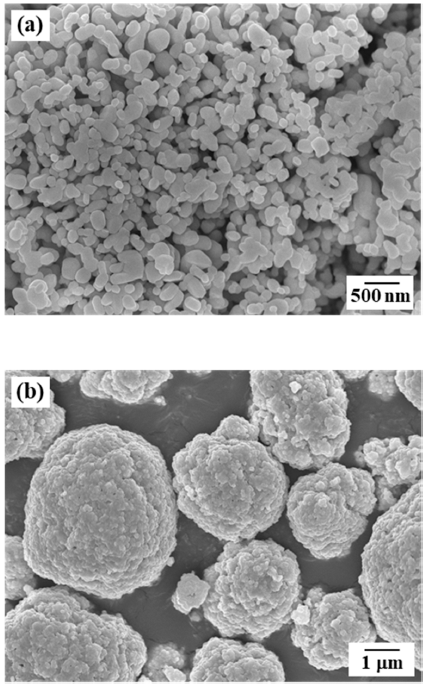
PDF In this study, freeze drying of a porous Ni with unidirectionally aligned pore channels is accomplished by using a NiO powder and camphene. Camphene slurries with NiO content of 5 and 10 vol% are prepared by mixing them with a small amount of dispersant at 50°C. Freezing of a slurry is performed at -25°C while the growth direction of the camphene is unidirectionally controlled. Pores are generated subsequently by sublimation of the camphene during drying in air for 48 h. The green bodies are hydrogen-reduced at 400°C and then sintered at 800°C and 900°C for 1 h. X-ray diffraction analysis reveals that the NiO powder is completely converted to the Ni phase without any reaction phases. The sintered samples show large pores that align parallel pores in the camphene growth direction as well as small pores in the internal walls of large pores. The size of large and small pores decreases with increasing powder content from 5 to 10 vol%. The influence of powder content on the pore structure is explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
Gyuhwi Lee, Ju-Yeon Han, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2020; 27(3): 193. CrossRef
- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
- [Korean]
- Fabrication of Porous Mo-Cu by Freeze Drying and Hydrogen Reduction of Metal Oxide Powders
- Hyunji Kang, Ju-Yeon Han, Sung-Tag Oh
- J Korean Powder Metall Inst. 2019;26(1):1-5. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.1

- 977 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, porous Mo-5 wt% Cu with unidirectionally aligned pores is prepared by freeze drying of camphene slurry with MoO3-CuO powders. Unidirectional freezing of camphene slurry with dispersion stability is conducted at -25°C, and pores in the frozen specimens are generated by sublimation of the camphene crystals. The green bodies are hydrogen-reduced at 750°C and sintered at 1000°C for 1 h. X-ray diffraction analysis reveals that MoO3-CuO composite powders are completely converted to a Mo-and-Cu phase without any reaction phases by hydrogen reduction. The sintered bodies with the Mo-Cu phase show large and aligned parallel pores to the camphene growth direction as well as small pores in the internal walls of large pores. The pore size and porosity decrease with increasing composite powder content from 5 to 10 vol%. The change of pore characteristics is explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Characteristic Evaluation of WC Hard Materials According to Ni Content Variation by a Pulsed Current Activated Sintering Process
Hyun-Kuk Park
Korean Journal of Materials Research.2020; 30(12): 672. CrossRef - Effect of α-lath size on the mechanical properties of Ti–6Al–4V using core time hydrogen heat treatment
Gye-Hoon Cho, Jung-Min Oh, Hanjung Kwon, Jae-Won Lim
Materials Science and Technology.2020; 36(7): 858. CrossRef
- Characteristic Evaluation of WC Hard Materials According to Ni Content Variation by a Pulsed Current Activated Sintering Process
- [Korean]
- Fabrication of Mo-Cu Powders by Ball Milling and Hydrogen Reduction of MoO3-CuO Powder Mixtures
- Hyunji Kang, Sung-Tag Oh
- J Korean Powder Metall Inst. 2018;25(4):322-326. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.322
- 913 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
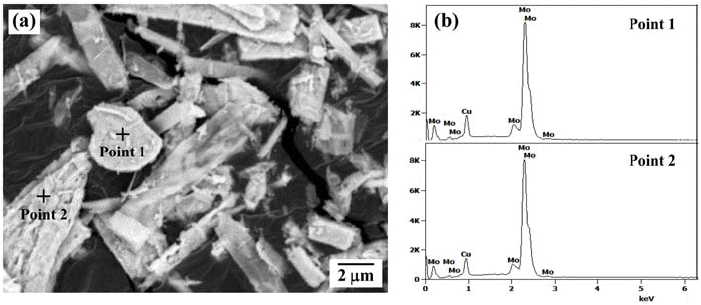
PDF The hydrogen reduction behavior of MoO3-CuO powder mixture for the synthesis of homogeneous Mo-20 wt% Cu composite powder is investigated. The reduction behavior of ball-milled powder mixture is analyzed by XRD and temperature programmed reduction method at various heating rates in Ar-10% H2 atmosphere. The XRD analysis of the heat-treated powder at 300°C shows Cu, MoO3, and Cu2MoO5 phases. In contrast, the powder mixture heated at 400°C is composed of Cu and MoO2 phases. The hydrogen reduction kinetic is evaluated by the amount of peak shift with heating rates. The activation energies for the reduction, estimated by the slope of the Kissinger plot, are measured as 112.2 kJ/mol and 65.2 kJ/mol, depending on the reduction steps from CuO to Cu and from MoO3 to MoO2, respectively. The measured activation energy for the reduction of MoO3 is explained by the effect of pre-reduced Cu particles. The powder mixture, hydrogen-reduced at 700°C, shows the dispersion of nano-sized Cu agglomerates on the surface of Mo powders.
-
Citations
Citations to this article as recorded by- Synthesis of Mo-Cu nanocomposite powder by hydrogen reduction of copper nitrate coated MoO3 powder mixture
Ji Won Choi, Ji Young Kim, Youngmin Kim, Eui Seon Lee, Sung-Tag Oh
Materials Letters.2024; 377: 137565. CrossRef - Fabrication of Porous Mo-Cu by Freeze Drying and Hydrogen Reduction of Metal Oxide Powders
Hyunji Kang, Ju-Yeon Han, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 1. CrossRef - Hydrogen Reduction Behavior and Microstructure Characteristics of Ball-milled CuO-Co3O4 Powder Mixtures
Ju-Yeon Han, Gyuhwi Lee, Hyunji Kang, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2019; 26(5): 410. CrossRef
- Synthesis of Mo-Cu nanocomposite powder by hydrogen reduction of copper nitrate coated MoO3 powder mixture
- [Korean]
- Recent Developments in H2 Production Photoelectrochemical Electrode Materials by Atomic Layer Deposition
- Jeong Hwan Han
- J Korean Powder Metall Inst. 2018;25(1):60-68. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.60
- 807 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The design and fabrication of photoelectrochemical (PEC) electrodes for efficient water splitting is important for developing a sustainable hydrogen evolution system. Among various development approaches for PEC electrodes, the chemical vapor deposition method of atomic layer deposition (ALD), based on self-limiting surface reactions, has attracted attention because it allows precise thickness and composition control as well as conformal coating on various substrates. In this study, recent research progress in improving PEC performance using ALD coating methods is discussed, including 3D and heterojunction-structured PEC electrodes, ALD coatings of noble metals, and the use of sulfide materials as co-catalysts. The enhanced long-term stability of PEC cells by ALD-deposited protecting layers is also reviewed. ALD provides multiple routes to develop improved hydrogen evolution PEC cells.
-
Citations
Citations to this article as recorded by- Improved Interface and Electrical Properties by Inserting an Ultrathin SiO2 Buffer Layer in the Al2O3/Si Heterojunction
Doyeon Kim, Jae‐Young Choi, Sang Wook Ryu, Woo‐Byoung Kim
Advanced Functional Materials.2019;[Epub] CrossRef
- Improved Interface and Electrical Properties by Inserting an Ultrathin SiO2 Buffer Layer in the Al2O3/Si Heterojunction
- [Korean]
- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
- Hyunji Kang, Sung Hyun Park, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(6):472-476. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.472

- 605 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF Porous W-10 wt% Ti alloys are prepared by freeze-drying a WO3-TiH2/camphene slurry, using a sintering process. X-ray diffraction analysis of the heat-treated powder in an argon atmosphere shows the WO3 peak of the starting powder and reaction-phase peaks such as WO2.9, WO2, and TiO2 peaks. In contrast, a powder mixture heated in a hydrogen atmosphere is composed of the W and TiW phases. The formation of reaction phases that are dependent on the atmosphere is explained by a thermodynamic consideration of the reduction behavior of WO3 and the dehydrogenation reaction of TiH2. To fabricate a porous W-Ti alloy, the camphene slurry is frozen at -30°C, and pores are generated in the frozen specimens by the sublimation of camphene while drying in air. The green body is hydrogen-reduced and sintered at 1000°C for 1 h. The sintered sample prepared by freeze-drying the camphene slurry shows large and aligned parallel pores in the camphene growth direction, and small pores in the internal walls of the large pores. The strut between large pores consists of very fine particles with partial necking between them.
- [Korean]
- Effect of Powder Mixing Process on the Characteristics of Hybrid Structure Tungsten Powders with Nano-Micro Size
- Na-Yeon Kwon, Young-Keun Jeong, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(5):384-388. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.384

- 877 View
- 3 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF The effect of the mixing method on the characteristics of hybrid-structure W powder with nano and micro sizes is investigated. Fine WO3 powders with sizes of ~0.6 μm, prepared by ball milling for 10 h, are mixed with pure W powder with sizes of 12 μm by various mixing process. In the case of simple mixing with ball-milled WO3 and micro sized W powders, WO3 particles are locally present in the form of agglomerates in the surface of large W powders, but in the case of ball milling, a relatively uniform distribution of WO3 particles is exhibited. The microstructural observation reveals that the ball milled WO3 powder, heat-treated at 750°C for 1 h in a hydrogen atmosphere, is fine W particles of ~200 nm or less. The powder mixture prepared by simple mixing and hydrogen reduction exhibits the formation of coarse W particles with agglomeration of the micro sized W powder on the surface. Conversely, in the powder mixture fabricated by ball milling and hydrogen reduction, a uniform distribution of fine W particles forming nano-micro sized hybrid structure is observed.
-
Citations
Citations to this article as recorded by- The Efficiency of Radiation Shielding Sheet to Reduce Radiation Exposure during C-arm Fluoroscopy
Hosang Jeon, Won Chul Shin, Hee Yun Seol, Yongkan Ki, Kyeong Baek Kim, Ki Seok Choo, Sang Don Lee, Suk-Woong Kang
Journal of the Korean Fracture Society.2023; 36(4): 111. CrossRef - Microstructure and Sintering Behavior of Fine Tungsten Powders Synthesized by Ultrasonic Spray Pyrolysis
Hyeonhui Jo, Jeong Hyun Kim, Young-In Lee, Young-Keun Jeong, Sung-Tag Oh
Korean Journal of Metals and Materials.2021; 59(5): 289. CrossRef - Facile phosphorus-embedding into SnS2 using a high-energy ball mill to improve the surface kinetics of P-SnS2 anodes for a Li-ion battery
Hongsuk Choi, Seungmin Lee, KwangSup Eom
Applied Surface Science.2019; 466: 578. CrossRef - Hydrogen reduction behavior and microstructural characteristics of WO3 and WO3-NiO powders
Hyunji Kang, Young-Keun Jeong, Sung-Tag Oh
International Journal of Refractory Metals and Hard Materials.2019; 80: 69. CrossRef - Fabrication of Densified W-Ti by Reaction Treatment and Spark Plasma Sintering of WO3-TiH2 Powder Mixtures
Hyunji Kang, Heun Joo Kim, Ju-Yeon Han, Yunju Lee, Young-Keun Jeong, Sung-Tag Oh
Korean Journal of Materials Research.2018; 28(9): 511. CrossRef
- The Efficiency of Radiation Shielding Sheet to Reduce Radiation Exposure during C-arm Fluoroscopy
- [Korean]
- Effect of Heat Treatment Temperature and Atmosphere on the Microstructure of TiH2-WO3 Powder Mixtures
- Han-Eol Lee, Yeon Su Kim, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(1):41-45. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.41

- 836 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The effects of the heat treatment temperature and of the atmosphere on the dehydrogenation and hydrogen reduction of ball-milled TiH2-WO3 powder mixtures are investigated for the synthesis of Ti-W powders with controlled microstructure. Homogeneously mixed powders with refined TiH2 particles are successfully prepared by ball milling for 24 h. X-ray diffraction (XRD) analyses show that the powder mixture heat-treated in Ar atmosphere is composed of Ti, Ti2O, and W phases, regardless of the heat treatment temperature. However, XRD results for the powder mixture, heat-treated at 600°C in a hydrogen atmosphere, show TiH2 and TiH peaks as well as reaction phase peaks of Ti oxides and W, while the powder mixture heat-treated at 900°C exhibits only XRD peaks attributed to Ti oxides and W. The formation behavior of the reaction phases that are dependent on the heat treatment temperature and on the atmosphere is explained by thermodynamic considerations for the dehydrogenation reaction of TiH2, the hydrogen reduction of WO3 and the partial oxidation of dehydrogenated Ti.
-
Citations
Citations to this article as recorded by- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
Hyunji Kang, Sung Hyun Park, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 472. CrossRef
- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
- [Korean]
- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
- Ki Cheol Jeon, Sung Hyun Park, Na-Yeon Kwon, Sung-Tag Oh
- J Korean Powder Metall Inst. 2016;23(4):303-306. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.303
- 903 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF An optimum route to synthesize Ti-Mo system powders is investigated by analyzing the effect of the heat treatment atmosphere on the formation of the reaction phase by dehydrogenation and hydrogen reduction of ball-milled TiH2-MoO3 powder mixtures. Homogeneous powder mixtures with refined particles are prepared by ball milling for 24 h. XRD analysis of the heat-treated powder in a hydrogen atmosphere shows TiH2 and MoO3 peaks in the initial powders as well as the peaks corresponding to the reaction phase species, such as TiH0.7, TiO, MoO2, Mo. In contrast, powder mixtures heated in an argon atmosphere are composed of Ti, TiO, Mo and MoO3 phases. The formation of reaction phases dependent on the atmosphere is explained by the partial pressure of H2 and the reaction temperature, based on thermodynamic considerations for the dehydrogenation reaction of TiH2 and the reduction behavior of MoO3.
-
Citations
Citations to this article as recorded by- Effect of titanium addition on mechanical properties of Mo-Si-B alloys
Won June Choi, Seung Yeong Lee, Chun Woong Park, Jung Hyo Park, Jong Min Byun, Young Do Kim
International Journal of Refractory Metals and Hard Materials.2019; 80: 238. CrossRef
- Effect of titanium addition on mechanical properties of Mo-Si-B alloys
- [English]
- Effects of Hydrogen Reduction in Microstructure, Mechanical and Thermoelectric Properties of Gas Atomized
n -type Bi2Te2.7 Se0.3 Material - Pradip Rimal, Sang-Min Yoon, Eun-Bin Kim, Chul-Hee Lee, Soon-Jik Hong
- J Korean Powder Metall Inst. 2016;23(2):126-131. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.126

- 938 View
- 5 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF The recent rise in applications of thermoelectric materials has attracted interest in studies toward the fabrication of thermoelectric materials using mass production techniques. In this study, we successfully fabricate
n -type Bi2Te2.7Se0.3 material by a combination of mass production powder metallurgy techniques, gas atomization, and spark plasma sintering. In addition, to examine the effects of hydrogen reduction in the microstructure, the thermoelectric and mechanical properties are measured and analyzed. Here, almost 60% of the oxygen content of the powder are eliminated after hydrogen reduction for 4 h at 360°C. Micrographs of the powder show that the reduced powder had a comparatively clean surface and larger grain sizes than unreduced powder. The density of the consolidated bulk using as-atomized powder and reduced atomized powder exceeds 99%. The thermoelectric power factor of the sample prepared by reduction of powder is 20% better than that of the sample prepared using unreduced powder.-
Citations
Citations to this article as recorded by- Tuning of power factor in bismuth selenide through Sn/Te co doping for low temperature thermoelectric applications
Ganesh Shridhar Hegde, Ashwatha Narayana Prabhu, Ramakrishna Nayak, C. F. Yang, Y. K. Kuo
Applied Physics A.2024;[Epub] CrossRef - Enhancing thermoelectric performance of K-doped polycrystalline SnSe through band engineering tuning and hydrogen reduction
Nan Xin, Yifei Li, Guihua Tang, Longyun Shen
Journal of Alloys and Compounds.2022; 899: 163358. CrossRef - The effect of powder pre-treatment on the mechanical and thermoelectric properties of spark plasma sintered N-type bismuth telluride
Ahmed A. Abdelnabi, Vickram Lakhian, Joseph R. McDermid, James S. Cotton
Journal of Alloys and Compounds.2021; 874: 159782. CrossRef - Investigation of Spark Plasma Sintering Temperature on Microstructure and Thermoelectric Properties of p-type Bi-Sb-Te alloys
Jin-Koo Han, Dong-won Shin, Babu Madavali, Soon-Jik Hong
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 115. CrossRef - The Preparation and Growth Mechanism of the Recovered Bi2Te3 Particles with Respect to Surfactants
Hyeongsub So, Eunpil Song, Yong-Ho Choa, Kun-Jae Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 141. CrossRef - Enhanced thermoelectric cooling properties of Bi2Te3−xSex alloys fabricated by combining casting, milling and spark plasma sintering
Seung Tek Han, Pradip Rimal, Chul Hee Lee, Hyo-Seob Kim, Yongho Sohn, Soon-Jik Hong
Intermetallics.2016; 78: 42. CrossRef
- Tuning of power factor in bismuth selenide through Sn/Te co doping for low temperature thermoelectric applications
- [Korean]
- Effect of Freezing and Sintering Condition of CuO-SnO2/Camphene Slurries on the Pore Structure of Porous Cu-Sn
- Joo-Hyung Kim, Sung-Tag Oh, Chang-Yong Hyun
- J Korean Powder Metall Inst. 2016;23(1):49-53. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.49
- 703 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The present study demonstrates the effect of freezing conditions on the pore structure of porous Cu-10 wt.% Sn prepared by freeze drying of CuO-SnO2/camphene slurry. Mixtures of CuO and SnO2 powders are prepared by ball milling for 10 h. Camphene slurries with 10 vol.% of CuO-SnO2 are unidirectionally frozen in a mold maintained at a temperature of -30°C for 1 and 24 h, respectively. Pores are generated by the sublimation of camphene at room temperature. After hydrogen reduction and sintering at 650°C for 2 h, the green body of the CuO-SnO2 is completely converted into porous Cu-Sn alloy. Microstructural observation reveals that the sintered samples have large pores which are aligned parallel to the camphene growth direction. The size of the large pores increases from 150 to 300 μm with an increase in the holding time. Also, the internal walls of the large pores contain relatively small pores whose size increases with the holding time. The change in pore structure is explained by the growth behavior of the camphene crystals and rearrangement of the solid particles during the freezing process.
-
Citations
Citations to this article as recorded by- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
Jae-Hun Jeong, Sung-Tag Oh, Chang-Yong Hyun
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 6. CrossRef - Fabrication of Al2O3 Dispersed Porous Cu by Freeze Drying of CuO-Al2O3/Camphene Slurry
Hyunji Kang, Doh-Hyung Riu, Sung-Tag Oh
journal of Korean Powder Metallurgy Institute.2018; 25(1): 25. CrossRef - Porous W-Ni Alloys Synthesized from Camphene/WO3-NiO Slurry by Freeze Drying and Heat Treatment in Hydrogen Atmosphere
Sung Hyun Park, Seong-Min Park, So-Jeong Park, Bo-Yeong Park, Sung-Tag Oh
Korean Journal of Materials Research.2018; 28(2): 108. CrossRef
- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
- [Korean]
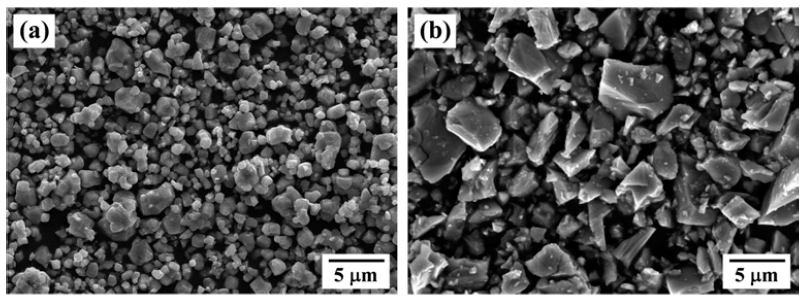
- Synthesis of NiTi Alloy Powder by the Reaction of NiO-TiH2 Mixing Powders
- Ki Cheol Jeon, Han-Eol Lee, Da-Mi Yim, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(4):266-270. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.266
- 813 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The synthesis of NiTi alloy powders by hydrogen reduction and dehydrogenation process of NiO and TiH2 powder mixtures is investigated. Mixtures of NiO and TiH2 powders are prepared by simple mixing for 1 h or ball milling for 24 h. Simple-mixed mixture shows that fine NiO particles are homogeneously coated on the surface of TiH2 powders, whereas ball milled one exhibits the morphology with mixing of fine NiO and TiH2 particles. Thermogravimetric analysis in hydrogen atmosphere reveals that the NiO and TiH2 phase are changed to metallic Ni and Ti in the temperature range of 260 to 290°C and 553 to 639°C, respectively. In the simple-mixed powders by heat-up to 700°C, agglomerates with solid particles and solidified liquid phase are observed, and the size of agglomerates is increased at 1000°C. From the XRD analysis, the presence of liquid phase is explained by the formation and melting of NiTi2 intermetallic compound due to an exothermic reaction between Ni and Ti. The simple-mixed powders, heated to 1000°C, lead to the formation of NiTi phase but additional Ni-, Ti-rich and Ti-oxide phases. In contrast, the microstructure of ball-milled powders is characterized by the neck-grown particles, forming Ni3Ti, Ti-oxide and unreacted Ni phase.
-
Citations
Citations to this article as recorded by- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
Ki Cheol Jeon, Sung Hyun Park, Na-Yeon Kwon, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 303. CrossRef
- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
- [Korean]
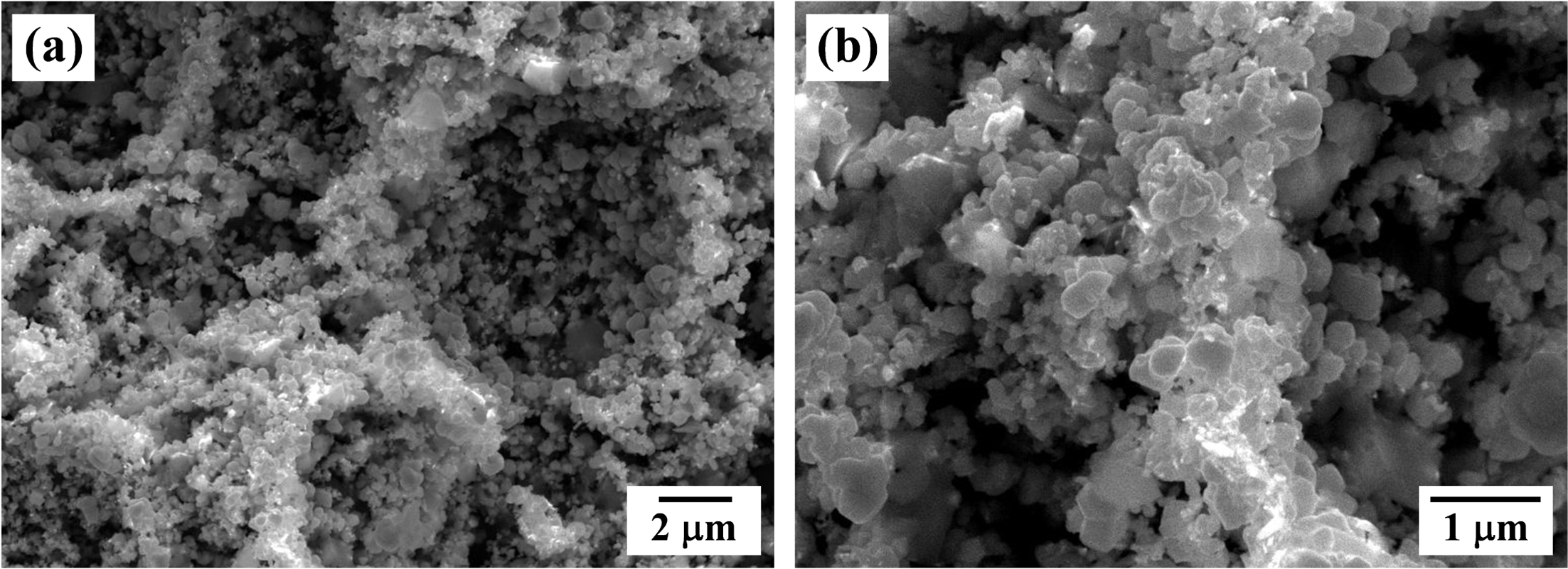
- Fabrication of Porous W by Heat Treatment of Pore Forming Agent of PMMA and WO3 Powder Compacts
- Ki Cheol Jeon, Young Do Kim, Myung-Jin Suk, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(2):129-133. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.129
- 1,558 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous W with controlled pore structure was fabricated by thermal decomposition and hydrogen reduction process of PMMA beads and WO3 powder compacts. The PMMA sizes of 8 and 50 μm were used as pore forming agent for fabricating the porous W. The WO3 powder compacts with 20 and 70 vol% PMMA were prepared by uniaxial pressing and sintered for 2 h at 1200°C in hydrogen atmosphere. TGA analysis revealed that the PMMA was decomposed at about 400°C and WO3 was reduced to metallic W at 800°C. Large pores in the sintered specimens were formed by thermal decomposition of spherical PMMA, and their size was increased with increase in PMMA size and the amount of PMMA addition. Also the pore shape was changed from spherical to irregular form with increasing PMMA contents due to the agglomeration of PMMA in the powder mixing process.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
Young-Sang Cho, Nahee Ku, Young-Seok Kim
JOURNAL OF CHEMICAL ENGINEERING OF JAPAN.2019; 52(2): 194. CrossRef
- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
- [Korean]
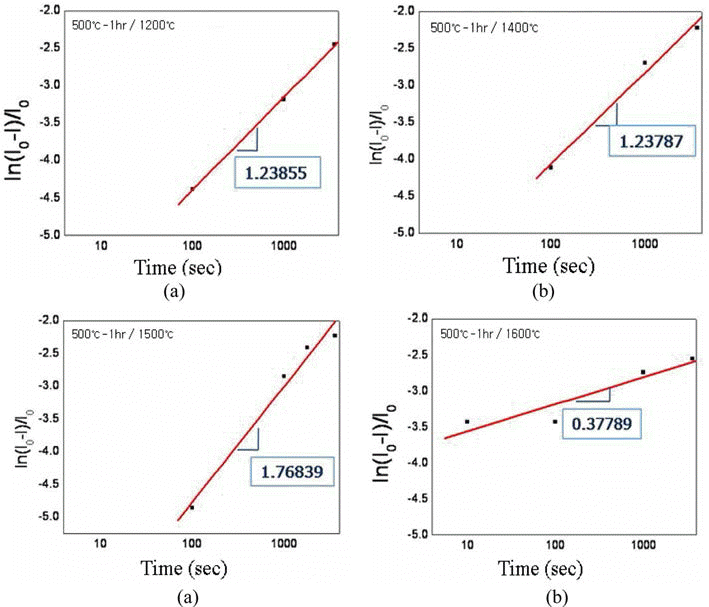
- Effect of Oxygen Content in the Tungsten Powder Fabricated by Electrical Explosion of Wire Method on the Behavior of Spark-Plasma Sintering
- Cheol-Hee Kim, Seong Lee, Byung-Kee Kim, Ji Soon Kim
- J Korean Powder Metall Inst. 2014;21(6):447-453. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.447
- 641 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Effect of oxygen content in the ultrafine tungsten powder fabricated by electrical explosion of wire method on the behvior of spark plasma sintering was investigated. The initial oxygen content of 6.5 wt% of as-fabricated tungsten powder was reduced to 2.3 and 0.7 wt% for the powders which were reduction-treated at 400°C for 2 hour and at 500°C for 1h in hydrogen atmosphere, respectively. The reduction-treated tungsten powders were spark-plasma sintered at 1200-1600°C for 100-3600 sec. with applied pressure of 50 MPa under vacuum of 0.133 Pa. Maximun sindered density of 97% relative density was obtained under the condition of 1600°C for 1h from the tungsten powder with 0.7 wt% oxygen. Sintering activation energy of 95.85 kJ/mol−1 was obtained, which is remarkably smaller than the reported ones of 380~460 kJ/mol−1 for pressureless sintering of micron-scale tungsten powders.
-
Citations
Citations to this article as recorded by- Effect of Powder Mixing Process on the Characteristics of Hybrid Structure Tungsten Powders with Nano-Micro Size
Na-Yeon Kwon, Young-Keun Jeong, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2017; 24(5): 384. CrossRef
- Effect of Powder Mixing Process on the Characteristics of Hybrid Structure Tungsten Powders with Nano-Micro Size
- [Korean]
- Hydrogen Reduction Behavior of Oxide Scale in Water-atomized Iron Powder
- Hea-Min Shin, Kyeong-Ho Baik
- J Korean Powder Metall Inst. 2014;21(6):422-428. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.422
- 1,131 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, the reduction kinetics and behaviors of oxides in the water-atomized iron powder have been evaluated as a function of temperature ranging 850-1000°C in hydrogen environment, and compared to the reduction behaviors of individual iron oxides including Fe2O3, Fe3O4 and FeO. The water-atomized iron powder contained a significant amount of iron oxides, mainly Fe3O4 and FeO, which were formed as a partially-continuous surface layer and an inner inclusion. During hydrogen reduction, a significant weight loss in the iron powder occurred in the initial stage of 10 min by the reduction of surface oxides, and then further reduction underwent slowly with increasing time. A higher temperature in the hydrogen reduction promoted a high purity of iron powder, but no significant change in the reduction occurred above 950°C. Sequence reduction process by an alternating environment of hydrogen and inert gases effectively removed the oxide scale in the iron powder, which lowered reduction temperature and/or shortened reduction time.
-
Citations
Citations to this article as recorded by- Carbon Co-Deposition During Gas Reduction of Water-Atomized Fe-Cr-Mo Powder
B. Ali, S.H. Choi, S.J. Seo, D.Y. Maeng, C.G. Lee, T.S. Kim, K.T. Park
Archives of Metallurgy and Materials.2017; 62(2): 1119. CrossRef
- Carbon Co-Deposition During Gas Reduction of Water-Atomized Fe-Cr-Mo Powder
- [Korean]
- Analysis of the Change in Microstructures of Nano Copper Powders During the Hydrogen Reduction using X-ray Diffraction Patterns and Transmission Electron Microscope, and the Mechanical Property of Compacted Powders
- Dong-Hyun Ahn, Dong Jun Lee, Wooyeol Kim, Lee Ju Park, Hyoung Seop Kim
- J Korean Powder Metall Inst. 2014;21(3):207-214. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.207

- 634 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this study, nano-scale copper powders were reduction treated in a hydrogen atmosphere at the relatively high temperature of 350°C in order to eliminate surface oxide layers, which are the main obstacles for fabricating a nano/ultrafine grained bulk parts from the nano-scale powders. The changes in composition and microstructure before and after the hydrogen reduction treatment were evaluated by analyzing X-ray diffraction (XRD) line profile patterns using the convolutional multiple whole profile (CMWP) procedure. In order to confirm the result from the XRD line profile analysis, transmitted electron microscope observations were performed on the specimen of the hydrogen reduction treated powders fabricated using a focused ion beam process. A quasi-statically compacted specimen from the nanoscale powders was produced and Vickers micro-hardness was measured to verify the potential of the powders as the basis for a bulk nano/ultrafine grained material. Although the bonding between particles and the growth in size of the particles occurred, crystallites retained their nano-scale size evaluated using the XRD results. The hardness results demonstrate the usefulness of the powders for a nano/ultrafine grained material, once a good consolidation of powders is achieved.
- [Korean]
- Fabrication of Porous Cu-Ni by Freeze Drying and Hydrogen Reduction of CuO-NiO Powder Mixture
- Han Gil Seo, Sung-Tag Oh
- J Korean Powder Metall Inst. 2014;21(1):34-38. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.34

- 885 View
- 0 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Cu-Ni alloys with unidirectionally aligned pores were prepared by freeze-drying process of CuO-NiO/camphene slurry. Camphene slurries with dispersion stability by the addition of oligomeric polyester were frozen at -25°C, and pores in the frozen specimens were generated by sublimation of the camphene during drying in air. The green bodies were hydrogen-reduced at 300°C and sintered at 850°C for 1 h. X-ray diffraction analysis revealed that CuO-NiO composite powders were completely converted to Cu-Ni alloy without any reaction phases by hydrogen reduction. The sintered samples showed large and aligned parallel pores to the camphene growth direction, and small pores in the internal wall of large pores. The pore size and porosity decreased with increase in CuO-NiO content from 5 to 10 vol%. The change of pore characteristics was explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
Eun Byeol Choi, Jong-Hyun Lee
Applied Surface Science.2017; 415: 67. CrossRef - Investigation for Microstructure and Hardness of Welded Zone of Cu-Ni Alloy using W92-Ni-Fe Sintering Tool
Tae-Jin Yoon, Sang-Won Park, Myung-Chang Kang, Joong-Suk Noh, Sung-Wook Chung, Chung-Yun Kang
Journal of Korean Powder Metallurgy Institute.2015; 22(3): 181. CrossRef - Controlling Structural and Electrical Properties of Pt Nanopowder-Dispersed SiO2Film
Jae Ho Lee, In Joo Shin, Sung Woo Lee, Hyeong Cheol Kim, Byung Joon Choi
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 355. CrossRef
- Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
TOP
 KPMI
KPMI


 First
First Prev
Prev


