Search
- Page Path
- HOME > Search
- [English]
- The Effect of a CNT/MnO2 Nanoparticle Composite–Based Multi-Shell Typed Electrode for a Fiber Supercapacitor (FSC)
- Yeonggwon Kim, Hyung Woo Lee
- J Powder Mater. 2025;32(1):30-36. Published online February 28, 2025
- DOI: https://doi.org/10.4150/jpm.2024.00416
- 1,123 View
- 26 Download
-
 Abstract
Abstract
 PDF
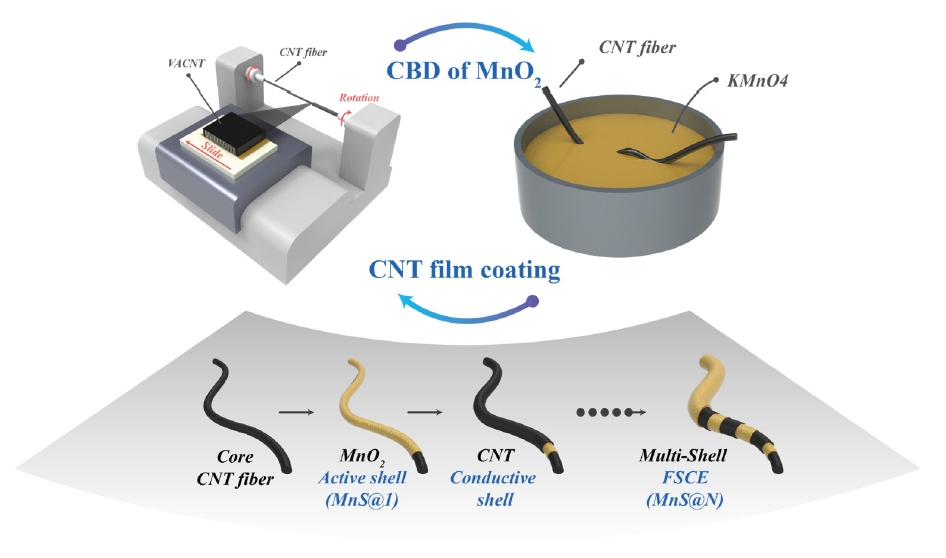
PDF - Fiber supercapacitors have attracted significant interest as potential textile energy storage devices due to their remarkable flexibility and rapid charge/discharge capabilities. This study describes the fabrication of a composite fiber supercapacitor (FSC) electrode through a multi-shell architecture, featuring layers of carbon nanotube (CNT) conductive shells and MnO₂ nanoparticle active shells. The number of layers was adjusted to assess their impact on FSC energy storage performance. Increasing the number of shells reduced electrode resistance and enhanced pseudocapacitive characteristics. Compared to the MnS@1 electrode, the MnS@5 electrode exhibited a high areal capacitance of 301.2 mF/cm², a 411% increase, but showed a higher charge transfer resistance (RCT) of 701.6 Ω. This is attributed to reduced ion diffusion and charge transfer ability resulting from the thicker multi-shell configuration. These results indicate that fine-tuning the quantity of shells is crucial for achieving an optimal balance between energy storage efficiency and stability.
- [English]
- Bandgap Tuning and Quenching Effects of In(Zn)P@ZnSe@ZnS Quantum Dots
- Sang Yeon Lee, Su Hyun Park, Gyungsu Byun, Chang-Yeoul Kim
- J Powder Mater. 2024;31(3):226-235. Published online June 27, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00003

- 3,016 View
- 44 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - InP quantum dot (QDs) have attracted researchers’ interest due to their applicability in quantum dot light-emitting displays (QLED) or biomarkers for detecting cancers or viruses. The surface or interface control of InP QD core/shell has substantially increased quantum efficiency, with a quantum yield of 100% reached by introducing HF to inhibit oxide generation. In this study, we focused on the control of bandgap energy of quantum dots by changing the Zn/(In+Zn) ratio in the In(Zn)P core. Zinc incorporation can change the photoluminescent light colors of green, yellow, orange, and red. Diluting a solution of as-synthesized QDs by more than 100 times did not show any quenching effects by the Förster resonance energy transfer phenomenon between neighboring QDs.
-
Citations
Citations to this article as recorded by- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
Hongcheng Yang, Junjie Hao, Mingyu Sun, Yujie Song, Kai Wang, Yujie Song, Xiao Wei Sun, Wenda Zhang
The Innovation.2026; 7(2): 101121. CrossRef
- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
- [Korean]
- Fabrication of Ti-Mo Core-shell Powder and Sintering Properties for Application as a Sputtering Target
- Won Hee Lee, Chun Woong Park, Heeyeon Kim, Yuncheol Ha, Jongmin Byun, Young Do Kim
- J Powder Mater. 2024;31(1):43-49. Published online February 28, 2024
- DOI: https://doi.org/10.4150/KPMI.2024.31.1.43
- 1,526 View
- 36 Download
- [Korean]
- Synthesis of Carbon Coated Nickel Cobalt Sulfide Yolk-shell Microsphere and Their Application as Anode Materials for Sodium Ion Batteries
- Hyo Yeong Seo, Gi Dae Park
- J Powder Mater. 2023;30(5):387-393. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.387
- 777 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF Transition metal chalcogenides are promising cathode materials for next-generation battery systems, particularly sodium-ion batteries. Ni3Co6S8-pitch-derived carbon composite microspheres with a yolk-shell structure (Ni3Co6S8@C-YS) were synthesized through a three-step process: spray pyrolysis, pitch coating, and post-heat treatment process. Ni3Co6S8@C-YS exhibited an impressive reversible capacity of 525.2 mA h g-1 at a current density of 0.5 A g-1 over 50 cycles when employed as an anode material for sodium-ion batteries. However, Ni3Co6S8 yolk shell nanopowder (Ni3Co6S8-YS) without pitch-derived carbon demonstrated a continuous decrease in capacity during charging and discharging. The superior sodium-ion storage properties of Ni3Co6S8@C-YS were attributed to the pitchderived carbon, which effectively adjusted the size and distribution of nanocrystals. The carbon-coated yolk-shell microspheres proposed here hold potential for various metal chalcogenide compounds and can be applied to various fields, including the energy storage field.
- [Korean]
- Recent progress on Performance Improvements of Thermoelectric Materials using Atomic Layer Deposition
- Seunghyeok Lee, Tae Joo Park, Seong Keun Kim
- J Powder Mater. 2022;29(1):56-62. Published online February 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.1.56
- 1,620 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF Atomic layer deposition (ALD) is a promising technology for the uniform deposition of thin films. ALD is based on a self-limiting mechanism, which can effectively deposit thin films on the surfaces of powders of various sizes. Numerous studies are underway to improve the performance of thermoelectric materials by forming core-shell structures in which various materials are deposited on the powder surface using ALD. Thermoelectric materials are especially relevant as clean energy storage materials due to their ability to interconvert between thermal and electrical energy by the Seebeck and Peltier effects. Herein, we introduce a surface and interface modification strategy based on ALD to control the performance of thermoelectric materials. We also discuss the properties of the interface between various deposition materials and thermoelectric materials.
- [Korean]
- Fabrication of TiO2 Coated Si Nano Particle using Silicon Sawing Sludge
- Dong Hyeok Seo, Hyeon Min Yim, Ho Yoon Na, Won Jin Kim, Ryun Na Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2021;28(5):423-428. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.423

- 678 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF Here, we report the development of a new and low-cost core-shell structure for lithium-ion battery anodes using silicon waste sludge and the Ti-ion complex. X-ray diffraction (XRD) confirmed the raw waste silicon sludge powder to be pure silicon without other metal impurities and the particle size distribution is measured to be from 200 nm to 3 μm by dynamic light scattering (DLS). As a result of pulverization by a planetary mill, the size of the single crystal according to the Scherrer formula is calculated to be 12.1 nm, but the average particle size of the agglomerate is measured to be 123.6 nm. A Si/TiO2 core-shell structure is formed using simple Ti complex ions, and the ratio of TiO2 peaks increased with an increase in the amount of Ti ions. Transmission electron microscopy (TEM) observations revealed that TiO2 coating on Si nanoparticles results in a Si-TiO2 core-shell structure. This result is expected to improve the stability and cycle of lithium-ion batteries as anodes.
- [Korean]
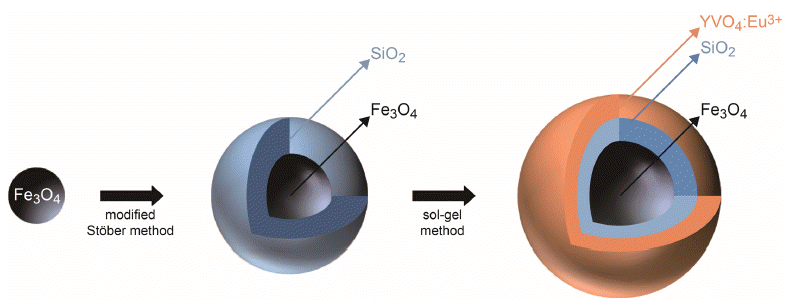
- Synthesis of the Multifunctional Core/Intermediate/Shell Nanoparticles: Tunable Magnetic and Photoluminescence Properties
- Mun-Kyoung Kim, Seyun Kim, Kyoung-Seok Moon, Weon Ho Shin, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2019;26(6):463-470. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.463
- 739 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Fe3O4/SiO2/YVO4:Eu3+ multifunctional nanoparticles are successfully synthesized by facile stepwise sol-gel processes. The multifunctional nanoparticles show a spherical shape with narrow size distribution (approximately 40 nm) and the phosphor shells are well crystallized. The Eu3+ shows strong photoluminescence (red emission at 619 nm, absorbance at 290 nm) due to an effective energy transfer from the vanadate group to Eu. Core-shell structured multifunctional nanoparticles have superparamagnetic properties at 300 K. Furthermore, the core-shell nanoparticles have a quick response time for the external magnetic field. These results suggest that the photoluminescence and magnetic properties could be easily tuned by either varying the number of coating processes or changing the phosphor elements. The nanoparticles may have potential applications for appropriate fields such as laser systems, optical amplifiers, security systems, and drug delivery materials.
- [Korean]
- Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
- Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2018;25(5):420-425. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.420

- 922 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Core-shell structured nanoparticles are garnering attention because these nanoparticles are expected to have a wide range of applications. The objective of the present study is to improve the coating efficiency of gold shell formed on the surface of silica nanoparticles for SiO2@Au core-shell structure. For the efficient coating of gold shell, we attempt an in-situ synthesis method such that the nuclei of the gold nanoparticles are generated and grown on the surface of silica nanoparticles. This method can effectively form a gold shell as compared to the conventional method of attaching gold nanoparticles to silica particles. It is considered possible to form a dense gold shell because the problems caused by electrostatic repulsion between the gold nanoparticles in the conventional method are eliminated.
- [Korean]
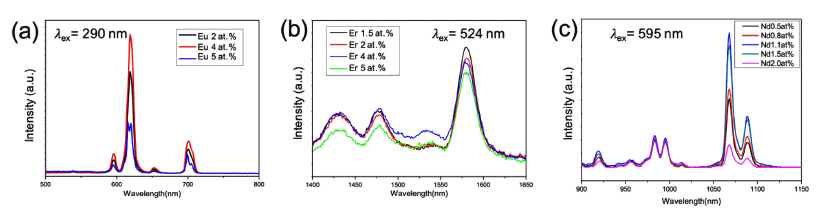
- Synthesis and Characterization of Core-Shell Silica-Phosphor Nanoparticles
via Sol-Gel Process - Weon Ho Shin, Seyun Kim, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2018;25(1):12-18. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.12
- 999 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Cost-effective functional phosphor nanoparticles are prepared by introducing low-cost SiO2 spheres to rareearth phosphor (YVO4:Eu3+, YVO4:Er3+, and YVO4:Nd3+) shells using a sol-gel synthetic method. These functional nanoparticles are characterized by X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, and general photoluminescence spectra. The SiO2 sphere occupying the interior of the conventional phosphor is advantageous in significantly reducing the cost of expensive rare-earth phosphor nanoparticles. The sol-gel process facilitates the core–shell structure formation; the rare-earth shell phosphor has strong interactions with chelating agents on the surfaces of SiO2 nanoparticles and thus forms layers of several nanometers in thickness. The photoluminescence wavelength is simply tuned by replacing the active materials of Eu3+, Er3+, and Nd3+. Moreover, the photoluminescent properties of the core–shell nanoparticles can be optimized by manipulating the specific contents of active materials in the phosphors. Our simple approach substitutes low-cost SiO2 for expensive rare-earth-based phosphor materials to realize cost-effective phosphor nanoparticles for various applications.
-
Citations
Citations to this article as recorded by- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
Woo Hyeong Sim, Seyun Kim, Weon Ho Shin, Hyung Mo Jeong
Korean Journal of Metals and Materials.2020; 58(2): 137. CrossRef
- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
- [Korean]
- Luminescence Properties of InP/ZnS Quantum Dots depending on InP Core synthesis Temperature
- Han Wook Seo, Da-Woon Jeong, Min Young Kim, Seoung Kyun Hyun, Ji Sun On, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(4):321-325. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.321
- 1,469 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, we investigate the optical properties of InP/ZnS core/shell quantum dots (QDs) by controlling the synthesis temperature of InP. The size of InP determined by the empirical formula tends to increase with temperature: the size of InP synthesized at 140oC and 220oC is 2.46 nm and 4.52 nm, respectively. However, the photoluminescence (PL) spectrum of InP is not observed because of the formation of defects on the InP surface. The growth of InP is observed during the deposition of the shell (ZnS) on the synthesized InP, which is ended up with green-red PL spectrum. We can adjust the PL spectrum and absorption spectrum of InP/ZnS by simply adjusting the core temperature. Thus, we conclude that there exists an optimum shell thickness for the QDs according to the size.
-
Citations
Citations to this article as recorded by- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- [English]
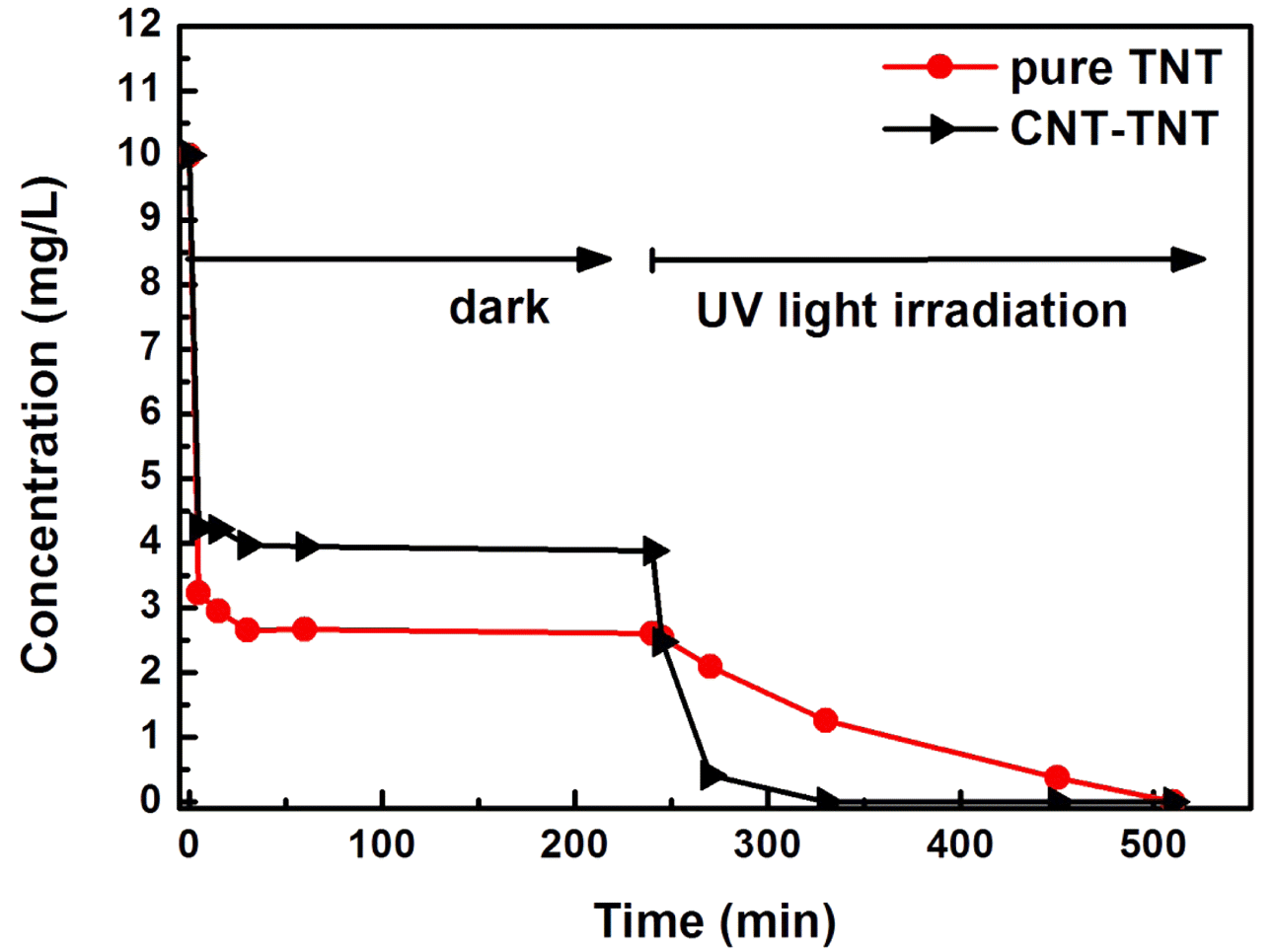
- The Synthesis and Photocatalytic activity of Carbon Nanotube-mixed TiO2 Nanotubes
- Chun Woong Park, Young Do Kim, Tohru Sekino, Se Hoon Kim
- J Korean Powder Metall Inst. 2017;24(4):279-284. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.279
- 1,516 View
- 11 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The formation mechanism and photocatalytic properties of a multiwalled carbon nanotube (MWCNT)/TiO2- based nanotube (TNTs) composite are investigated. The CNT/TNT composite is synthesized via a solution chemical route. It is confirmed that this 1-D nanotube composite has a core-shell nanotubular structure, where the TNT surrounds the CNT core. The photocatalytic activity investigated based on the methylene blue degradation test is superior to that of with pure TNT. The CNTs play two important roles in enhancing the photocatalytic activity. One is to act as a template to form the core-shell structure while titanate nanosheets are converted into nanotubes. The other is to act as an electron reservoir that facilitates charge separation and electron transfer from the TNT, thus decreasing the electronhole recombination efficiency.
-
Citations
Citations to this article as recorded by- Carbon-based photocatalysis in organic transformation
Md Razu Ahmed, Yuta Nishina
Bulletin of the Chemical Society of Japan.2026;[Epub] CrossRef - Low-Dimensional Carbon and Titania Nanotube Composites via a Solution Chemical Process and Their Nanostructural and Electrical Properties for Electrochemical Devices
Sunghun Eom, Sung Hun Cho, Tomoyo Goto, Myoung Pyo Chun, Tohru Sekino
ACS Applied Nano Materials.2019; 2(10): 6230. CrossRef
- Carbon-based photocatalysis in organic transformation
- [Korean]
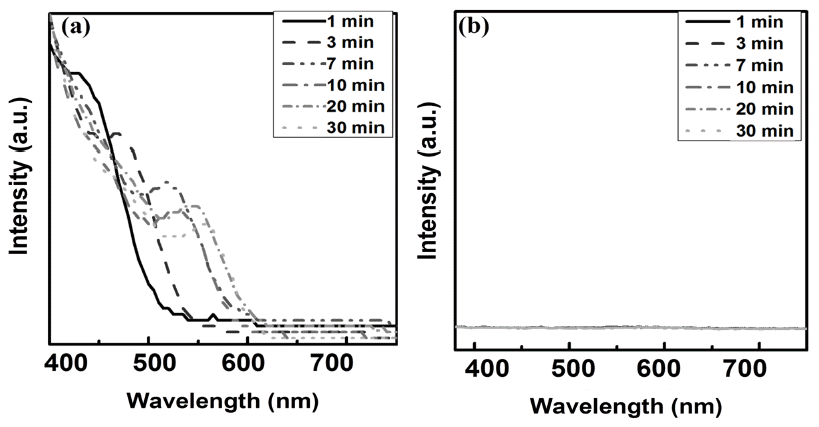
- Growth mechanism of InP and InP/ZnS synthesis using colloidal synthesis
- Han wook Seo, Da-woon Jeong, Bin Lee, Seoung kyun Hyun, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(1):6-10. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.6
- 1,446 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF This study investigates the main growth mechanism of InP during InP/ZnS reaction of quantum dots (QDs). The size of the InP core, considering a synthesis time of 1-30 min, increased from the initial 2.56 nm to 3.97 nm. As a result of applying the proposed particle growth model, the migration mechanism, with time index 7, was found to be the main reaction. In addition, after the removal of unreacted In and P precursors from bath, further InP growth (of up to 4.19 nm (5%)), was observed when ZnS was added. The full width at half maximum (FWHM) of the synthesized InP/ZnS quantum dots was found to be relatively uniform, measuring about 59 nm. However, kinetic growth mechanism provides limited information for InP / ZnS core shell QDs, because the surface state of InP changes with reaction time. Further study is necessary, in order to clearly determine the kinetic growth mechanism of InP / ZnS core shell QDs.
- [Korean]
- Synthesis and Characterization of Brilliant Yellow Color Pigments using α-FeOOH Nanorods
- JiYeon Yun, Ri Yu, YooJin Kim
- J Korean Powder Metall Inst. 2016;23(6):453-457. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.453

- 627 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this work, we synthesize brilliant yellow color α-FeOOH by controlling the rod length and core-shell structure. The characteristics of α-FeOOH nanorods are controlled by the reaction conditions. In particular, the length of the α-FeOOH rods depends on the concentration of the raw materials, such as the alkali solution. The length of the nanorods is adjusted from 68 nm to 1435 nm. Their yellowness gradually increases, with the highest b* value of 57 based on the International Commission on Illumination (CIE)
Lab system, by controlling the nanorod length. A high quality yellow color is obtained after formation of a silica coating on the α-FeOOH structure. The morphology and the coloration of the nal products are investigated in detail by X-ray diffraction, scanning electron microscopy, UV-vis spectroscopy, and the CIELab color parameter measurements.
- [English]
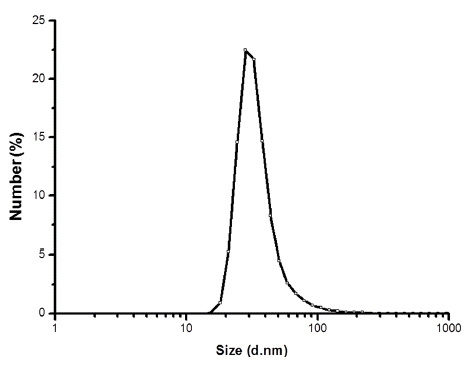
- Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
- A-Young Lee, Gwang-Yeob Lee, Hye-Ryeong Oh, Hyeon-Ah Kim, Song-Yi Kim, Min-Ha Lee
- J Korean Powder Metall Inst. 2016;23(6):409-413. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.409
- 928 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF Electrical wire explosion in liquid media is a promising method for producing metallic nanopowders. It is possible to obtain high-purity metallic nanoparticles and uniform-sized nanopowder with excellent dispersion stability using this electrical wire explosion method. In this study, Ni-Fe alloy nanopowders with core-shell structures are fabricated via the electrical explosion of Ni-Fe alloy wires 0.1 mm in diameter and 20 mm in length in de-ionized water. The size and shape of the powders are investigated by field-emission scanning electron microscopy, transmission electron microscopy, and laser particle size analysis. Phase analysis and grain size determination are conducted by X-ray diffraction. The result indicate that a core-shell structured Ni-Fe nanopowder is synthesized with an average particle size of approximately 28 nm, and nanosized Ni core particles are encapsulated by an Fe nanolayer.
- [Korean]
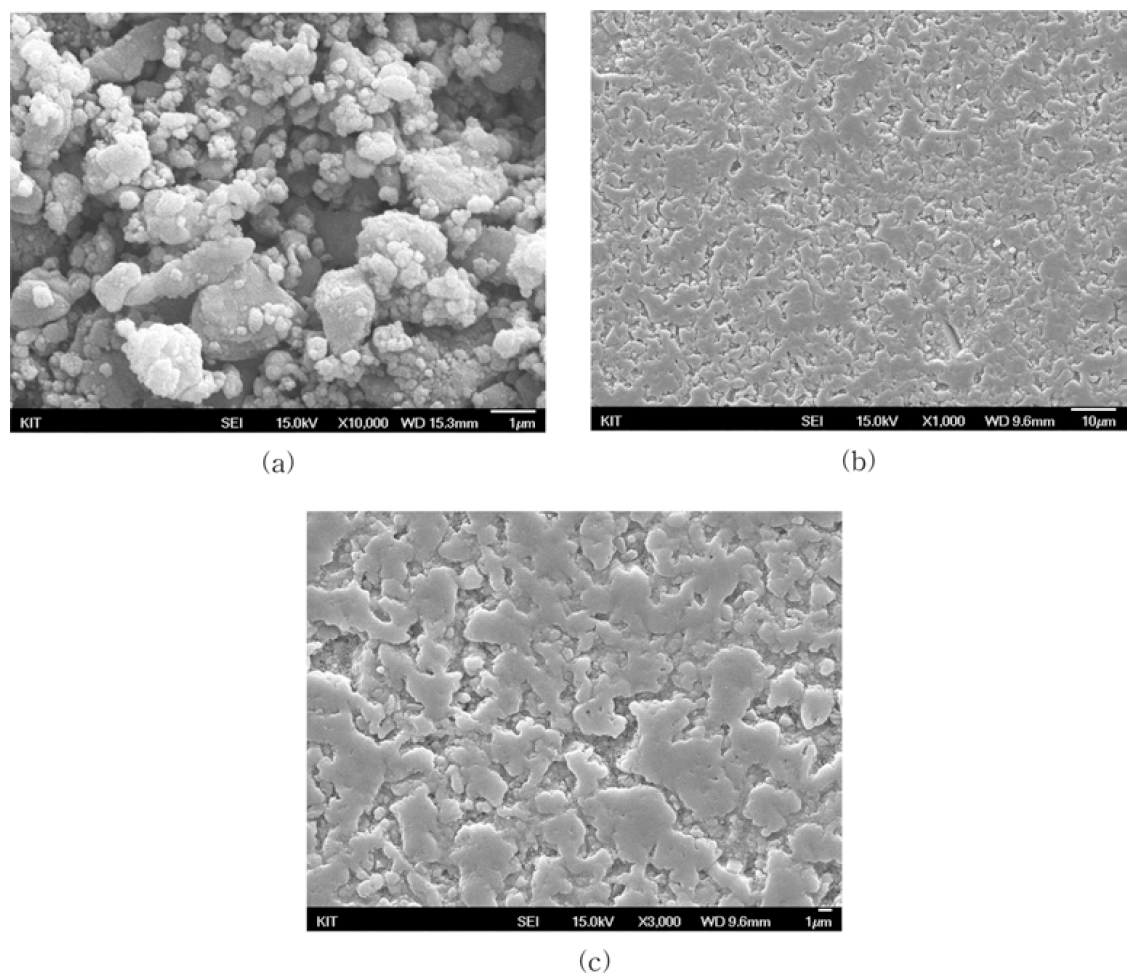
- Consolidation to Bulk Ceramic Bodies from Oyster Shell Powder
- Kyeong-Sik Cho, Hyun-Kwuon Lee, Jae Hong Min
- J Korean Powder Metall Inst. 2016;23(3):221-227. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.221
- 1,504 View
- 10 Download
-
 Abstract
Abstract
 PDF
PDF Waste oyster shells create several serious problems; however, only some parts of them are being utilized currently. The ideal solution would be to convert the waste shells into a product that is both environmentally beneficial and economically viable. An experimental study is carried out to investigate the recycling possibilities for oyster shell waste. Bulk ceramic bodies are produced from the oyster shell powder in three sequential processes. First, the shell powder is calcined to form calcium oxide CaO, which is then slaked by a slaking reaction with water to produce calcium hydroxide Ca(OH)2. Then, calcium hydroxide powder is formed by uniaxial pressing. Finally, the calcium hydroxide compact is reconverted to calcium carbonate via a carbonation reaction with carbon dioxide released from the shell powder bed during firing at 550°C. The bulk body obtained from waste oyster shells could be utilized as a marine structural porous material.
- [Korean]
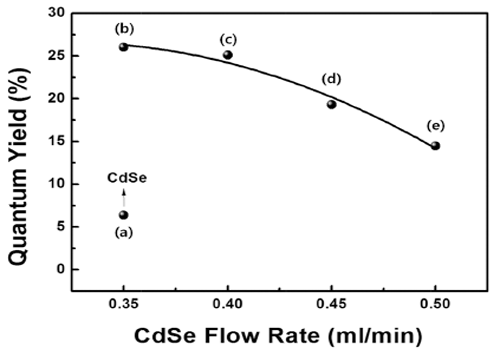
- Optical Characteristics of CdSe/ZnS Quantum Dot with Precursor Flow Rate Synthesized by using Microreactor
- Ji Young Park, Da-Woon Jeong, Won Ju, Han Wook Seo, Yong-Ho Choa, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(2):91-94. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.91
- 1,269 View
- 6 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF High-quality colloidal CdSe/ZnS (core/shell) is synthesized using a continuous microreactor. The particle size of the synthesized quantum dots (QDs) is a function of the precursor flow rate; as the precursor flow rate increases, the size of the QDs decreases and the band gap energy increases. The photoluminescence properties are found to depend strongly on the flow rate of the CdSe precursor owing to the change in the core size. In addition, a gradual shift in the maximum luminescent wave (λmax) to shorter wavelengths (blue shift) is found owing to the decrease in the QD size in accordance with the quantum confinement effect. The ZnS shell decreases the surface defect concentration of CdSe. It also lowers the thermal energy dissipation by increasing the concentration of recombination. Thus, a relatively high emission and quantum yield occur because of an increase in the optical energy emitted at equal concentration. In addition, the maximum quantum yield is derived for process conditions of 0.35 ml/min and is related to the optimum thickness of the shell material.
-
Citations
Citations to this article as recorded by- Quantum materials made in microfluidics - critical review and perspective
M. Wojnicki, V. Hessel
Chemical Engineering Journal.2022; 438: 135616. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- Quantum materials made in microfluidics - critical review and perspective
- [Korean]
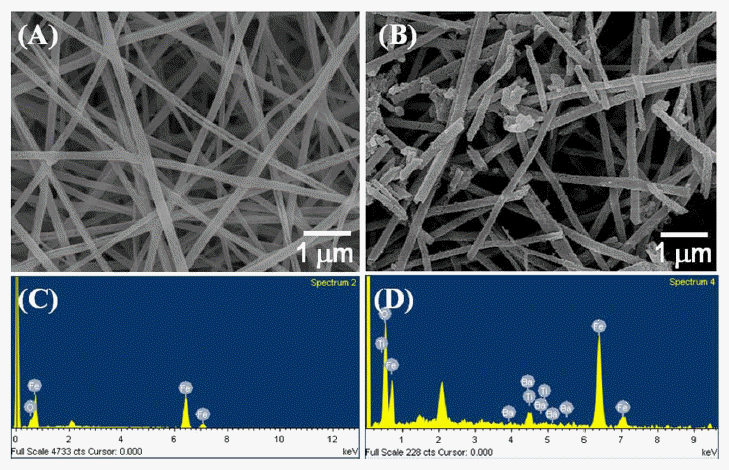
- Synthesis and Electromagnetic Wave Absorbing Property of BaTiO3@Fe Nanofibers with Core-Shell Structure
- Young-In Lee, Dae-Hwan Jang, Ki-Hoon Sung, Kyuman Lee, Yong-Ho Choa
- J Korean Powder Metall Inst. 2016;23(1):38-42. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.38
- 1,008 View
- 6 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF BaTiO3-coated Fe nanofibers are synthesized via a three-step process. α-Fe2O3 nanofibers with an average diameter of approximately 200 nm are first prepared using an electrospinning process followed by a calcination step. The BaTiO3 coating layer on the nanofiber is formed by a sol-gel process, and a thermal reduction process is then applied to the core-shell nanofiber to selectively reduce the α-Fe2O3 to Fe. The thickness of the BaTiO3 shell is controlled by varying the reaction time. To evaluate the electromagnetic (EM) wave-absorbing abilities of the BaTiO3@Fe nanofiber, epoxy-based composites containing the nanofibers are fabricated. The composites show excellent EM wave absorption properties where the power loss increases to the high frequency region without any degradation. Our results demonstrate that the BaTiO3@Fe nanofibers obtained in this work are attractive candidates for electromagnetic wave absorption applications.
-
Citations
Citations to this article as recorded by- Research on Electromagnetic Wave Absorption Based on Electrospinning Technology†
Baoding Li, Jing Qiao, Yue Liu, Haoyuan Tian, Wei Liu, Qilei Wu, Zhou Wang, Jiurong Liu, Zhihui Zeng
Chinese Journal of Chemistry.2024; 42(7): 777. CrossRef - Design, synthesis, and characterization of a porous ceramic-supported CeO2 nanocatalyst for CO -free H2 evolution
Jimin Lee, Minseob Lim, Tae Sung Kim, Kee-Ryung Park, Jong-Sik Lee, Hong-Baek Cho, Joo Hyun Park, Yong-Ho Choa
Applied Surface Science.2021; 548: 149198. CrossRef - Electromagnetic wave absorption properties of Fe/MgO composites synthesized by a simple ultrasonic spray pyrolysis method
Hyo-Ryoung Lim, Seung-Jae Jung, Tae-Yeon Hwang, Jimin Lee, Ki Hyeon Kim, Hong-baek Cho, Yong-Ho Choa
Applied Surface Science.2019; 473: 1009. CrossRef - Study on the Optimization of Reduction Conditions for Samarium-Cobalt Nanofiber Preparation
Jimin Lee, Jongryoul Kim, Yong-Ho Choa
Journal of Korean Powder Metallurgy Institute.2019; 26(4): 334. CrossRef
- Research on Electromagnetic Wave Absorption Based on Electrospinning Technology†
- [Korean]
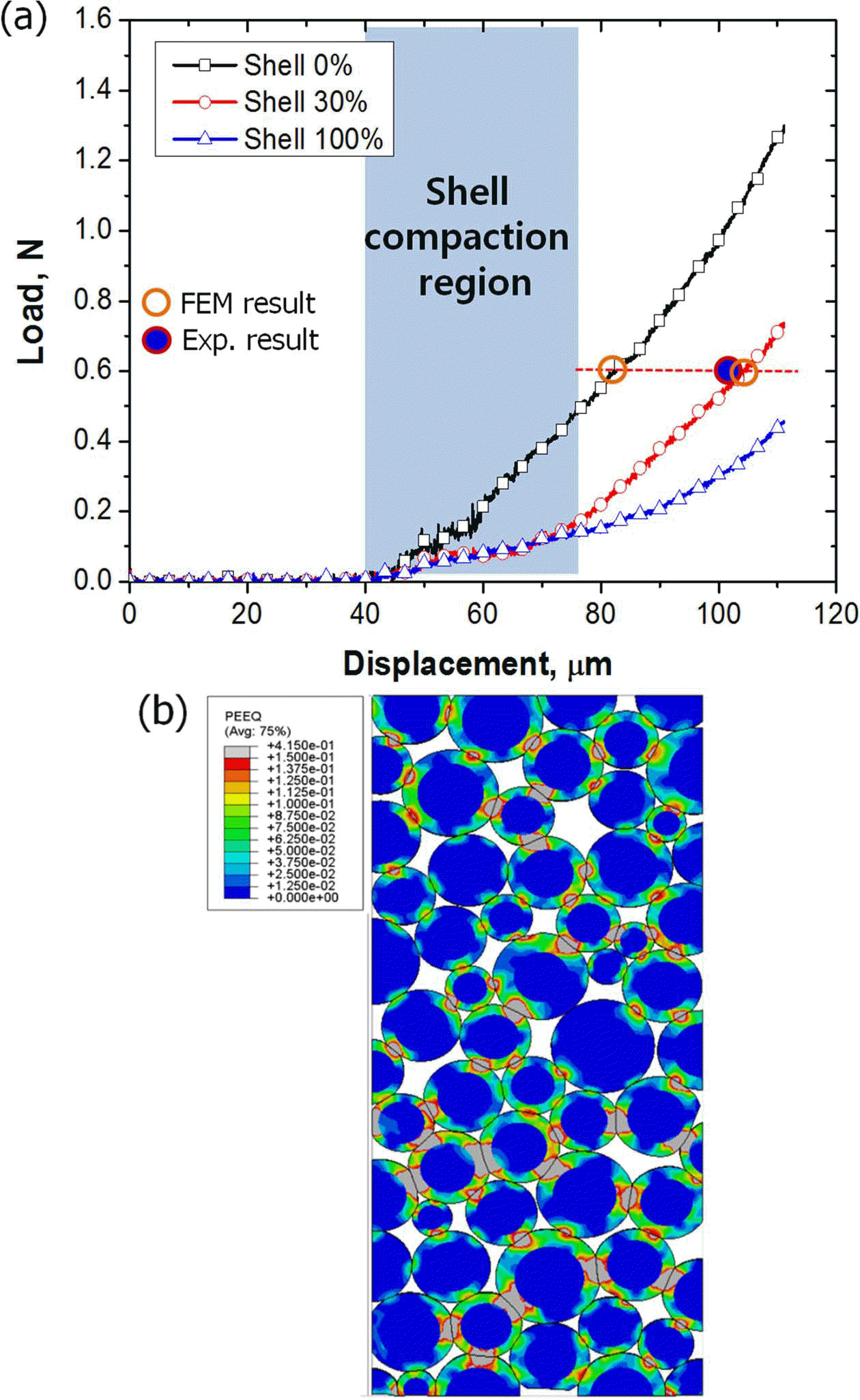
- Effect of Core-Shell Structure on Compaction Behavior of Harmonic Powder
- Soo-Hyun Joo, Hyo Wook Park, Soo Young Kang, Eon Sik Lee, Hee-Soo Kang, Hyong Seop Kim
- J Korean Powder Metall Inst. 2015;22(2):105-110. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.105
- 616 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF In this study, effect of core-shell structure on compaction behavior of harmonic powder is investigated. Harmonic powders are made by electroless plating method on Fe powders. Softer Cu shell encloses harder Fe core, and the average size of Fe core and thickness of Cu shell are 34.3 μm and 3.2 μm, respectively. The powder compaction procedure is processed with pressure of 600 MPa in a cylindrical die. Due to the low strength of Cu shell regions, the harmonic powders show better densification behavior compared with pure Fe powders. Finite element method (FEM) is performed to understand the roll of core-shell structure. Based on stress and strain distributions of FEM results, it is concluded that the early stage of powder compaction of harmonic powders mainly occurs at the shell region. FEM results also well predict porosity of compacted materials.
TOP
 KPMI
KPMI


 First
First Prev
Prev


