Search
- Page Path
- HOME > Search
- [Korean]
- Enhanced H2S Gas Sensing Using ZnO Porous Nanorod Synthesized via a Rotational Hydrothermal Method
- Jimyeong Park, Changyu Kim, Minseo Kim, Jiyeon Shin, Jae-Hyoung Lee, Myung Sik Choi
- J Powder Mater. 2025;32(5):406-415. Published online October 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00262
- 123 View
- 3 Download
-
 Abstract
Abstract
 PDF
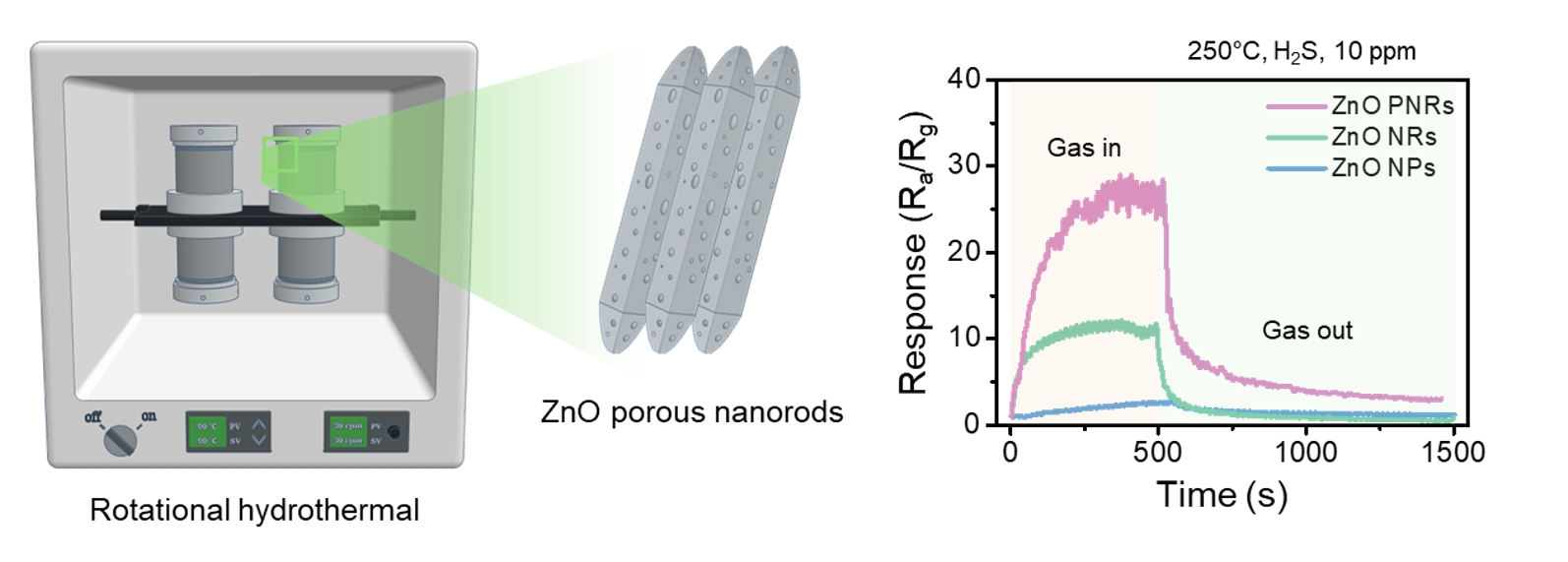
PDF - In this study, ZnO porous nanorods were synthesised using a rotational hydrothermal process, and their performance as hydrogen sulphide (H2S) gas sensors was analysed. Compared to commercial ZnO nanoparticles and conventionally hydrothermally synthesised ZnO nanorods, the ZnO porous nanorods exhibited a more uniform structure and improved crystal growth in the (002) plane, with surfaces rich in porosity and oxygen vacancies. These structural and chemical characteristics significantly improved the sensitivity toward H2S, showing high detection performance at 250°C across various concentrations of H2S gas. Additionally, the sensor demonstrated excellent selectivity against other gases such as C2H5OH, C6H6, C7H8, and NH3. This study indicated that the rotational hydrothermal process is an effective method for developing high-performance ZnO-based gas sensors and suggests its applicability to other metal oxide materials.
- [English]
- The Effect of TiO2 Addition on Low-temperature Sintering Behaviors in a SnO2-CoO-CuO System
- Jae-Sang Lee, Kyung-Sik Oh, Yeong-Kyeun Paek
- J Powder Mater. 2024;31(2):146-151. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00024

- 980 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - Pure SnO2 has proven very difficult to densify. This poor densification can be useful for the fabrication of SnO2 with a porous microstructure, which is used in electronic devices such as gas sensors. Most electronic devices based on SnO2 have a porous microstructure, with a porosity of > 40%. In pure SnO2, a high sintering temperature of approximately 1300C is required to obtain > 40% porosity. In an attempt to reduce the required sintering temperature, the present study investigated the low-temperature sinterability of a current system. With the addition of TiO2, the compositions of the samples were Sn1-xTixO2-CoO(0.3wt%)-CuO(2wt%) in the range of x ≤ 0.04. Compared to the samples without added TiO2, densification was shown to be improved when the samples were sintered at 950C. The dominant mass transport mechanism appears to be grain-boundary diffusion during heat treatment at 950C.
- [Korean]
- Fabrication of Porous Tungsten by Freeze Casting and Vacuum Drying of WO3/Tert-butyl Alcohol Slurry
- Youn Ji Heo, Eui Seon Lee, Sung-Tag Oh, Young-Keun Jeong
- J Powder Mater. 2022;29(2):118-122. Published online April 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.2.118

- 703 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The synthesis of porous W by freeze-casting and vacuum drying is investigated. Ball-milled WO3 powders and tert-butyl alcohol were used as the starting materials. The tert-butyl alcohol slurry is frozen at –25°C and dried under vacuum at –25 and –10°C. The dried bodies are hydrogen-reduced at 800°C and sintered at 1000°C. The XRD analysis shows that WO3 is completely reduced to W without any reaction phases. SEM observations reveal that the struts and pores aligned in the tert-butyl alcohol growth direction, and the change in the powder content and drying temperature affects the pore structure. Furthermore, the struts of the porous body fabricated under vacuum are thinner than those fabricated under atmospheric pressure. This behavior is explained by the growth mechanism of tert-butyl alcohol and rearrangement of the powders during solidification. These results suggest that the pore structure of a porous body can be controlled by the powder content, drying temperature, and pressure.
-
Citations
Citations to this article as recorded by-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
Ji Won Choi, Youngmin Kim, Ji Young Kim, Eui Seon Lee, Sung-Tag Oh
Powder Metallurgy.2025; 68(3): 283. CrossRef - Fabrication of Porous TiO2 with Aligned Pores Using Tert-Butyl Alcohol Based Freeze Casting
Eui Seon Lee, Sung-Tag Oh
Korean Journal of Metals and Materials.2024; 62(12): 929. CrossRef
-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
- [Korean]
- Effect of Freeze Drying Condition of WO3/Tert-Butyl Alcohol Slurry on the Microstructural Characteristics of Porous Body
- Eui Seon Lee, Youn Ji Heo, Myung-Jin Suk, Sung-Tag Oh
- J Korean Powder Metall Inst. 2021;28(4):331-335. Published online August 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.4.331

- 467 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF The effects of drying temperature on the microstructure of porous W fabricated by the freeze-casting process of tert-butyl alcohol slurry with WO3 powder was investigated. Green bodies were hydrogen-reduced at 800°C for 1 h and sintered at 1000°C for 6 h. X-ray diffraction analysis revealed that WO3 powders were completely converted to W without any reaction phases by hydrogen reduction. The sintered body showed pores aligned in the direction of tertbutyl alcohol growth, and the porosity and pore size decreased as the amount of WO3 increased from 5 to 10v ol%. As the drying temperature of the frozen body increased from -25°C to -10°C, the pore size and thickness of the struts increased. The change in microstructural characteristics based on the amount of powder added and the drying temperature was explained by the growth behavior of the freezing agent and the degree of rearrangement of the solid powder during the solidification of the slurry.
- [Korean]
- Effect of Tert-Butyl Alcohol Template on the Pore Structure of Porous Tungsten in Freeze Drying Process
- Eui Seon Lee, Youn Ji Heo, Yun Taek Ko, Jin Gyeong Park, Yong-Ho Choa, Sung-Tag Oh
- J Korean Powder Metall Inst. 2021;28(3):216-220. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.216

- 548 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The effect of tert-butyl alcohol (TBA) as a freezing solvent on the pore structure of a porous tungsten body prepared by freeze-drying is analyzed. TBA slurries with a WO3 content of 10 vol% are prepared by mixing with a small amount of dispersant and binder at 30°C. The slurries are frozen at -25°C, and pores are formed in the frozen specimens by the sublimation of TBA during drying in air. After hydrogen reduction at 800°C and sintering at 1000°C, the green body of WO3 is completely converted to porous W with various pore structures. Directional pores from the center of the specimen to the outside are observed in the sintered bodies because of the columnar growth of TBA. A decrease in pore directionality and porosity is observed in the specimens prepared by long-duration drying and sintering. The change in pore structure is explained by the growth of the freezing solvent and densification.
- [Korean]
- The Effects of Kaolin Addition on the Properties of Reticulated Porous Diatomite-kaolin Composites
- Chae-Young Lee, Sujin Lee, Jang-Hoon Ha, Jongman Lee, In-Hyuck Song, Kyoung-Seok Moon
- J Korean Powder Metall Inst. 2020;27(4):325-332. Published online August 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.4.325
- 771 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF In this study, the effects of kaolin addition on the properties of reticulated porous diatomite-kaolin composites are investigated. A reticulated porous diatomite-kaolin composite is prepared using the replica template method. The microstructure and pore characteristics of the reticulated porous diatomite-kaolin composites are analyzed by controlling the PPI value (45, 60, and 80 PPI) of the polyurethane foam (which are used as the polymer template), the ball-milling time (8 and 24 h), and the amount of kaolin (0–50 wt. %). The average pore size decreases as the amount of kaolin increases in the reticulated porous diatomite-kaolin composite. As the amount of kaolin increases, it can be determined that the amount of inter-connected pore channels is reduced because the plate-shaped kaolin particles connect the gaps between irregular diatomite particles. Consequently, a higher kaolin percentage affects the overall mechanical properties by improving the pore channel connectivity. The effect of kaolin addition on the basic properties of the reticulated porous diatomite-kaolin composite is further discussed with characterization data such as pore size distribution, scanning electron microscopy images, and compressive strength.
- [Korean]
- Freeze Drying Process and Pore Structure Characteristics of Porous Cu with Various Sublimable Vehicles
- Gyuhwi Lee, Sung-Tag Oh, Myung-Jin Suk, Young-Keun Jeong
- J Korean Powder Metall Inst. 2020;27(3):198-202. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.198
- 576 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF The effect of sublimable vehicles on the pore structure of Cu fabricated by freeze drying is investigated. The 5 vol% CuO-dispersed slurries with camphene and various camphor-naphthalene compositions are frozen in a Teflon mold at -25°C, followed by sublimation at room temperature. After hydrogen reduction at 300°C and sintering at 600 °C, the green bodies of CuO are completely converted to Cu with various pore structures. The sintered samples prepared using CuO/camphene slurries show large pores that are aligned parallel to the sublimable vehicle growth direction. In addition, a dense microstructure is observed in the bottom section of the specimen where the solidification heat was released, owing to the difference in the solidification behavior of the camphene crystals. The porous Cu shows different pore structures, such as dendritic, rod-like, and plate shaped, depending on the composition of the camphornaphthalene system. The change in pore structure is explained by the crystal growth behavior of primary camphor and eutectic and primary naphthalene.
- [Korean]
- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
- Gyuhwi Lee, Ju-Yeon Han, Sung-Tag Oh
- J Korean Powder Metall Inst. 2020;27(3):193-197. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.193
- 562 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Porous Cu-14 wt% Co with aligned pores is produced by a freeze drying and sintering process. Unidirectional freezing of camphene slurry with CuO-Co3O4 powders is conducted, and pores in the frozen specimens are generated by sublimation of the camphene crystals. The dried bodies are hydrogen-reduced at 500°C and sintered at 800°C for 1 h. The reduction behavior of the CuO-Co3O4 powder mixture is analyzed using a temperature-programmed reduction method in an Ar-10% H2 atmosphere. The sintered bodies show large and aligned parallel pores in the camphene growth direction. In addition, small pores are distributed around the internal walls of the large pores. The size and fraction of the pores decrease as the amount of solid powder added to the slurry increases. The change in pore characteristics according to the amount of the mixed powder is interpreted to be due to the rearrangement and accumulation behavior of the solid particles in the freezing process of the slurry.
- [Korean]
- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
- Jae-Hun Jeong, Sung-Tag Oh, Chang-Yong Hyun
- J Korean Powder Metall Inst. 2019;26(1):6-10. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.6
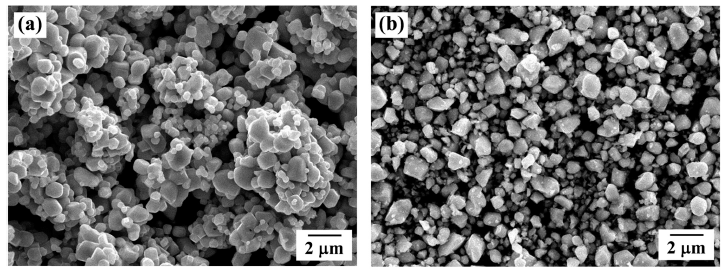
- 746 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, freeze drying of a porous Ni with unidirectionally aligned pore channels is accomplished by using a NiO powder and camphene. Camphene slurries with NiO content of 5 and 10 vol% are prepared by mixing them with a small amount of dispersant at 50°C. Freezing of a slurry is performed at -25°C while the growth direction of the camphene is unidirectionally controlled. Pores are generated subsequently by sublimation of the camphene during drying in air for 48 h. The green bodies are hydrogen-reduced at 400°C and then sintered at 800°C and 900°C for 1 h. X-ray diffraction analysis reveals that the NiO powder is completely converted to the Ni phase without any reaction phases. The sintered samples show large pores that align parallel pores in the camphene growth direction as well as small pores in the internal walls of large pores. The size of large and small pores decreases with increasing powder content from 5 to 10 vol%. The influence of powder content on the pore structure is explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
Gyuhwi Lee, Ju-Yeon Han, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2020; 27(3): 193. CrossRef
- Synthesis of Porous Cu-Co using Freeze Drying Process of Camphene Slurry with Oxide Composite Powders
- [Korean]
- Fabrication of Porous Mo-Cu by Freeze Drying and Hydrogen Reduction of Metal Oxide Powders
- Hyunji Kang, Ju-Yeon Han, Sung-Tag Oh
- J Korean Powder Metall Inst. 2019;26(1):1-5. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.1

- 774 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, porous Mo-5 wt% Cu with unidirectionally aligned pores is prepared by freeze drying of camphene slurry with MoO3-CuO powders. Unidirectional freezing of camphene slurry with dispersion stability is conducted at -25°C, and pores in the frozen specimens are generated by sublimation of the camphene crystals. The green bodies are hydrogen-reduced at 750°C and sintered at 1000°C for 1 h. X-ray diffraction analysis reveals that MoO3-CuO composite powders are completely converted to a Mo-and-Cu phase without any reaction phases by hydrogen reduction. The sintered bodies with the Mo-Cu phase show large and aligned parallel pores to the camphene growth direction as well as small pores in the internal walls of large pores. The pore size and porosity decrease with increasing composite powder content from 5 to 10 vol%. The change of pore characteristics is explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Characteristic Evaluation of WC Hard Materials According to Ni Content Variation by a Pulsed Current Activated Sintering Process
Hyun-Kuk Park
Korean Journal of Materials Research.2020; 30(12): 672. CrossRef - Effect of α-lath size on the mechanical properties of Ti–6Al–4V using core time hydrogen heat treatment
Gye-Hoon Cho, Jung-Min Oh, Hanjung Kwon, Jae-Won Lim
Materials Science and Technology.2020; 36(7): 858. CrossRef
- Characteristic Evaluation of WC Hard Materials According to Ni Content Variation by a Pulsed Current Activated Sintering Process
- [Korean]
- A Study on Pore Properties of SUS316L Powder Porous Metal Fabricated by Electrostatic Powder Coating Process
- Min-Jeong Lee, Yu-Jeong Yi, Hyeon-Ju Kim, Manho Park, Byoung-Kee Kim, Jung-Yeul Yun
- J Korean Powder Metall Inst. 2018;25(5):415-419. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.415
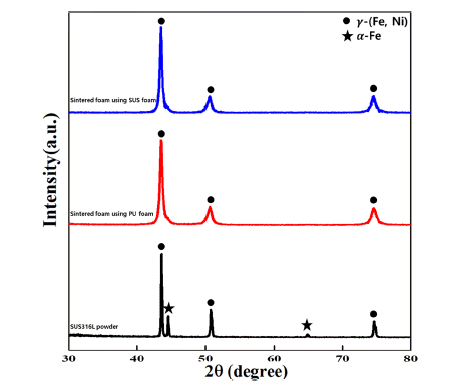
- 573 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous metals demonstrate not only excessively low densities, but also novel physical, thermal, mechanical, electrical, and acoustic properties. Thus, porous metals exhibit exceptional performance, which are useful for diesel particulate filters, heat exchangers, and noise absorbers. In this study, SUS316L foam with 90% porosity and 3,000 μm pore size is successfully manufactured using the electrostatic powder coating (ESPC) process. The mean size of SUS316L powders is approximately 12.33 μm. The pore properties are evaluated using SEM and Archimedes. As the quantity of powder coating increases, pore size decreases from 2,881 to 1,356 μm. Moreover, the strut thickness and apparent density increase from 423.7 to 898.3 μm and from 0.278 to 0.840 g/cm3, respectively. It demonstrates that pore properties of SUS316L powder porous metal are controllable by template type and quantity of powder coating.
-
Citations
Citations to this article as recorded by- Fabrication and Pore Characteristics of Metal Powder Filters with a Cross-Sealed Honeycomb Shape Using Material Extrusion Additive Manufacturing
Minji Kim, Min-Jeong Lee, Su-Jin Yun, Poong-Yeon Kim, Hyeon Ju Kim, Juyong Kim, Jung Woo Lee, Jung-Yeul Yun
Journal of Powder Materials.2025; 32(4): 299. CrossRef
- Fabrication and Pore Characteristics of Metal Powder Filters with a Cross-Sealed Honeycomb Shape Using Material Extrusion Additive Manufacturing
- [English]
- Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
- Bong Gill Choi, MinHo Yang
- J Korean Powder Metall Inst. 2018;25(3):203-207. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2017.25.3.203
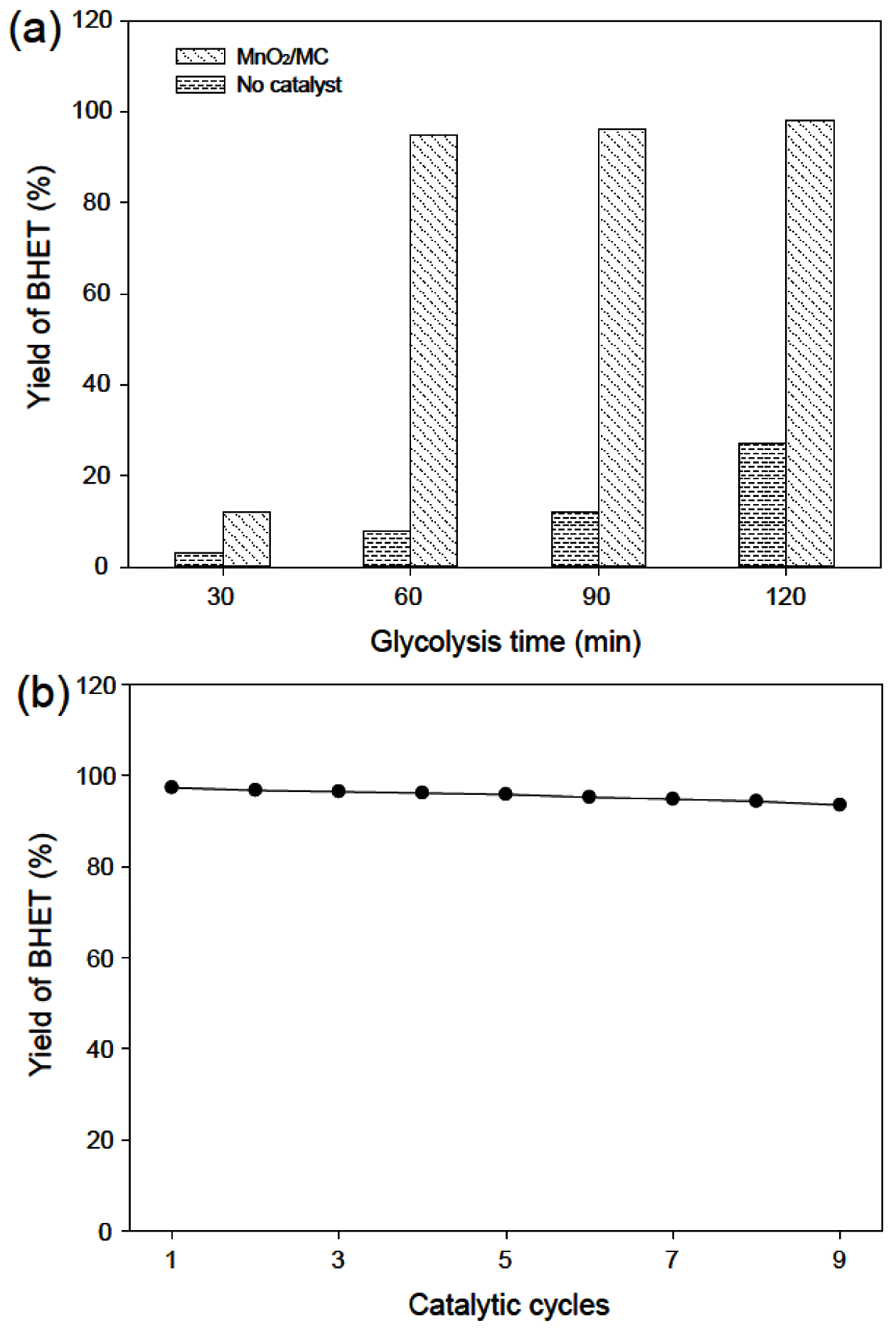
- 489 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Plastic pollution is threatening human health and ecosystems, resulting in one of the biggest challenges that humanity has ever faced. Therefore, this study focuses on the preparation of macroporous carbon from biowaste (MC)-supported manganese oxide (MnO2) as an efficient, reusable, and robust catalyst for the recycling of poly(ethylene terephthalate) (PET) waste. As-prepared MnO2/MC composites have a hierarchical pore network and a large surface area (376.16 m2/g) with a narrow size distribution. MnO2/MC shows a maximum yield (98%) of bis(2-hydroxyethyl)terephthalate (BHET) after glycolysis reaction for 120 min. Furthermore, MnO2/MC can be reused at least nine times with a negligible decrease in BHET yield. Based on this remarkable catalytic performance, we expect that MnO2-based heterogeneous catalysts have the potential to be introduced into the PET recycling industry.
- [Korean]
- High-Contrast Electrochromism of Porous Tungsten Oxide Thin Films Prepared by Electrodeposition
- Sung-Hyeok Park, Ho-Jin Mo, Jae-Keun Lim, Sang-Gwon Kim, Jae-Hyo Choi, Seung-Hyun Lee, Se-Hwa Jang, Kyung-Ho Cha, Yoon-Chae Nah
- J Korean Powder Metall Inst. 2018;25(1):7-11. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.7
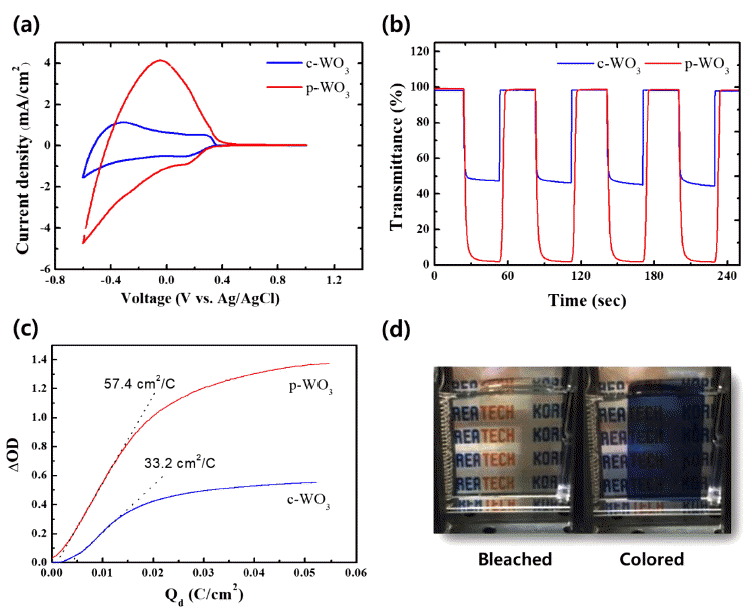
- 807 View
- 16 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, we synthesize tungsten oxide thin films by electrodeposition and characterize their electrochromic properties. Depending on the deposition modes, compact and porous tungsten oxide films are fabricated on a transparent indium tin oxide (ITO) substrate. The morphology and crystal structure of the electrodeposited tungsten oxide thin films are investigated by scanning electron microscopy (SEM) and X-ray diffraction (XRD). X-ray photoelectron spectroscopy is employed to verify the chemical composition and the oxidation state of the films. Compared to the compact tungsten oxides, the porous films show superior electrochemical activities with higher reversibility during electrochemical reactions. Furthermore, they exhibit very high color contrast (97.0%) and switching speed (3.1 and 3.2 s). The outstanding electrochromic performances of the porous tungsten oxide thin films are mainly attributed to the porous structure, which facilitates ion intercalation/deintercalation during electrochemical reactions.
-
Citations
Citations to this article as recorded by- A fast-response electrochromic device based on a composite gel film comprising triphenylamine derivatives and WO3
Xuejian Zhang, Jinming Zeng, Zipeng Xu, Mimi Zhu, Ping Liu
New Journal of Chemistry.2021; 45(12): 5503. CrossRef
- A fast-response electrochromic device based on a composite gel film comprising triphenylamine derivatives and WO3
- [Korean]
- Fabrication of Al2O3 Dispersed Porous Cu by Freeze Drying of CuO-Al2O3/Camphene Slurry
- Hyunji Kang, Doh-Hyung Riu, Sung-Tag Oh
- J Korean Powder Metall Inst. 2018;25(1):25-29. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.25

- 487 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Porous Cu with a dispersion of nanoscale Al2O3 particles is fabricated by freeze-drying CuO-Al2O3/camphene slurry and sintering. Camphene slurries with CuO-Al2O3 contents of 5 and 10 vol% are unidirectionally frozen at -30°C, and pores are generated in the frozen specimens by camphene sublimation during air drying. The green bodies are sintered for 1 h at 700°C and 800°C in H2 atmosphere. The sintered samples show large pores of 100 μm in average size aligned parallel to the camphene growth direction. The internal walls of the large pores feature relatively small pores of ~10 μm in size. The size of the large pores decreases with increasing CuO-Al2O3 content by the changing degree of powder rearrangement in the slurry. The size of the small pores decreases with increasing sintering temperature. Microstructural analysis reveals that 100-nm Al2O3 particles are homogeneously dispersed in the Cu matrix. These results suggest that a porous composite body with aligned large pores could be fabricated by a freeze-drying and H2 reducing process.
- [Korean]
- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
- Hyunji Kang, Sung Hyun Park, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(6):472-476. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.472

- 469 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF Porous W-10 wt% Ti alloys are prepared by freeze-drying a WO3-TiH2/camphene slurry, using a sintering process. X-ray diffraction analysis of the heat-treated powder in an argon atmosphere shows the WO3 peak of the starting powder and reaction-phase peaks such as WO2.9, WO2, and TiO2 peaks. In contrast, a powder mixture heated in a hydrogen atmosphere is composed of the W and TiW phases. The formation of reaction phases that are dependent on the atmosphere is explained by a thermodynamic consideration of the reduction behavior of WO3 and the dehydrogenation reaction of TiH2. To fabricate a porous W-Ti alloy, the camphene slurry is frozen at -30°C, and pores are generated in the frozen specimens by the sublimation of camphene while drying in air. The green body is hydrogen-reduced and sintered at 1000°C for 1 h. The sintered sample prepared by freeze-drying the camphene slurry shows large and aligned parallel pores in the camphene growth direction, and small pores in the internal walls of the large pores. The strut between large pores consists of very fine particles with partial necking between them.
- [English]
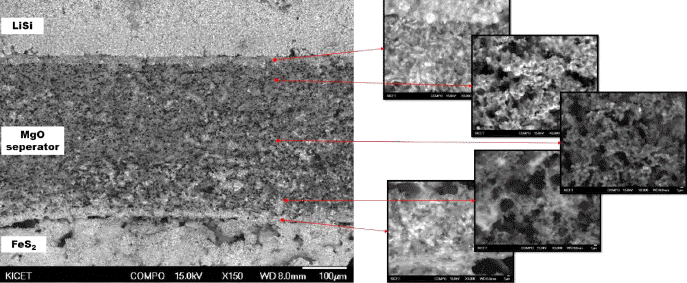
- Fabrication and Characterization of Thermal Battery using Porous MgO Separator Infiltrated with Li based Molten Salts
- Kyungho Kim, Sungmin Lee, Chae-Nam Im, Seung-Ho Kang, Hae-Won Cheong, Yoonsoo Han
- J Korean Powder Metall Inst. 2017;24(5):364-369. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.364
- 617 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF Ceramic powder, such as MgO, is added as a binder to prepare the green compacts of molten salts of an electrolyte for a thermal battery. Despite the addition of a binder, when the thickness of the electrolyte decreases to improve the battery performance, the problem with the unintentional short circuit between the anode and cathode still remains. To improve the current powder molding method, a new type of electrolyte separator with porous MgO preforms is prepared and characteristics of the thermal battery are evaluated. A Spherical PMMA polymer powder is added as a pore-forming agent in the MgO powder, and an organic binder is used to prepare slurry appropriate for tape casting. A porous MgO preform with 300 μm thickness is prepared through a binder burnout and sintering process. The particle size of the starting MgO powder has an effect, not on the porosity of the porous MgO preform, but on the battery characteristics. The porosity of the porous MgO preforms is controlled from 60 to 75% using a pore-forming agent. The batteries prepared using various porosities of preforms show a performance equal to or higher than that of the pellet-shaped battery prepared by the conventional powder molding method.
- [Korean]
- Effects of Porous Microstructure on the Electrochemical Properties of Si-Ge-Al Base Anode Materials for Li-ion Rechargeable Batteries
- Chung Rae Cho, Myeong Geun Kim, Keun Yong Sohn, Won-Wook Park
- J Korean Powder Metall Inst. 2017;24(1):24-28. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.24
- 496 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Silicon alloys are considered promising anode active materials to replace Li-ion batteries by graphite powder, because they have a relatively high capacity of up to 4200 mAh/g, and are environmentally friendly and inexpensive ECO-materials. However, its poor charge/discharge properties, induced by cracking during cycles, constitute their most serious problem as anode electrode. In order to solve these problems, Si-Ge-Al alloys with porous structure are designed as anode alloy powders, to improve cycling stability. The alloys are melt-spun to obtain the rapidly solidified ribbons, and then ball-milled to make fine powders. The powders are etched using 1 M HCl solution, which gives the powders a porous structure by removing the element Al. Subsequently, in this study, the microstructures and the characteristics of the etched powders are evaluated for application as anode materials. As a result, the etched porous powder shows better electrochemical properties than as-milled Si-Ge-Al powder.
- [Korean]
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2016;23(3):235-239. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.235
- 366 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF This study is performed to fabricate a Ti porous body by freeze drying process using titanium hydride (TiH2) powder and camphene. Then, the Ti porous body is employed to synthesize carbon nanotubes (CNTs) using thermal catalytic chemical vapor deposition (CCVD) with Fe catalyst and methane (CH4) gas to increase the specific surface area. The synthesized Ti porous body has 100 μm-sized macropores and 10-30 μm-sized micropores. The synthesized CNTs have random directions and are entangled with adjacent CNTs. The CNTs have a bamboo-like structure, and their average diameter is about 50 nm. The Fe nano-particles observed at the tip of the CNTs indicate that the tip growth model is applicable. The specific surface area of the CNT-coated Ti porous body is about 20 times larger than that of the raw Ti porous body. These CNT-coated Ti porous bodies are expected to be used as filters or catalyst supports.
- [Korean]
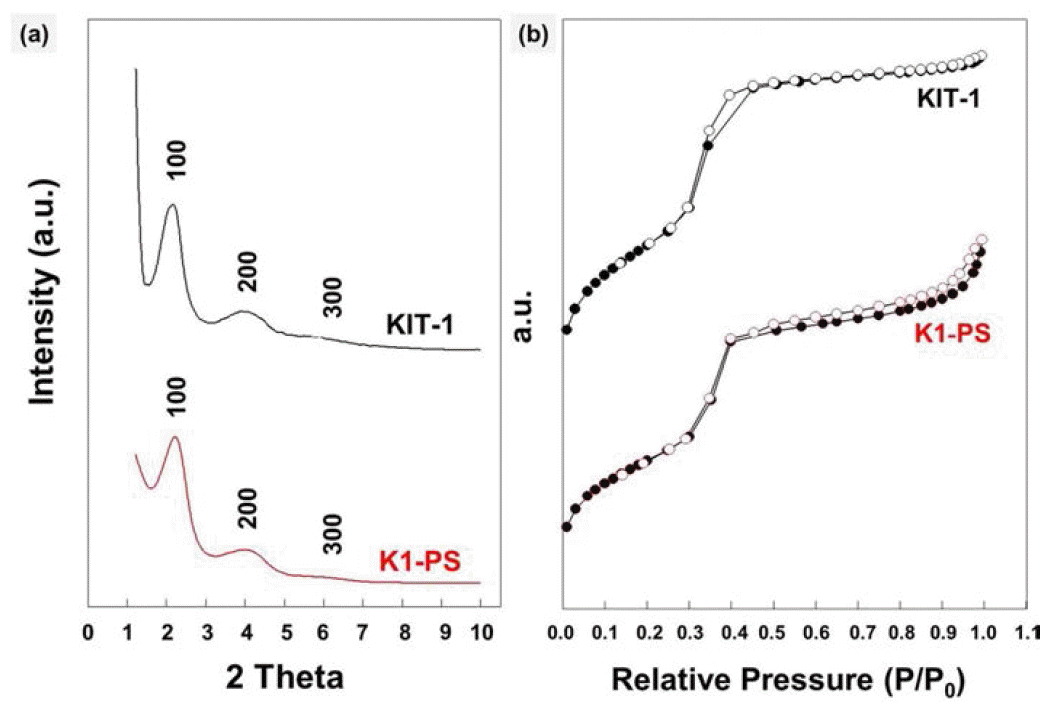
- Synthesis of KIT-1 Mesoporous Silicates Showing Two Different Macrosporous Strucrtues; Inverse-opal or Hollow Structures
- Youn-Kyoung Baek, Jung-Goo Lee, Young Kuk Kim
- J Korean Powder Metall Inst. 2016;23(3):189-194. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.189
- 486 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF We report a facile method for preparing KIT-1 mesoporous silicates with two different macroporous structures by dual templating. As a template for macropores, polystyrene (PS) beads are assembled into uniform three dimensional arrays by ice templating, i.e., by growing ice crystals during the freezing process of the particle suspension. Then, the polymeric templates are directly introduced into the precursor-gel solution with cationic surfactants for templating the mesopores, which is followed by hydrothermal crystallization and calcination. Later, by burning out the PS beads and the surfactants, KIT-1 mesoporous silicates with macropores are produced in a powder form. The macroporous structures of the silicates can be controlled by changing the amount of EDTANa4 salt under the same templating conditions using the PS beads and inverse-opal or hollow structures can be obtained. This strategy to prepare mesoporous powders with controllable macrostructures is potentially useful for various applications especially those dealing with bulky molecules such as, catalysis, separation, drug carriers and environmental adsorbents.
- [Korean]
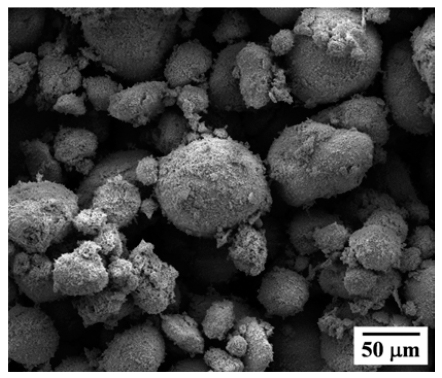
- Effect of Freezing and Sintering Condition of CuO-SnO2/Camphene Slurries on the Pore Structure of Porous Cu-Sn
- Joo-Hyung Kim, Sung-Tag Oh, Chang-Yong Hyun
- J Korean Powder Metall Inst. 2016;23(1):49-53. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.49
- 554 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The present study demonstrates the effect of freezing conditions on the pore structure of porous Cu-10 wt.% Sn prepared by freeze drying of CuO-SnO2/camphene slurry. Mixtures of CuO and SnO2 powders are prepared by ball milling for 10 h. Camphene slurries with 10 vol.% of CuO-SnO2 are unidirectionally frozen in a mold maintained at a temperature of -30°C for 1 and 24 h, respectively. Pores are generated by the sublimation of camphene at room temperature. After hydrogen reduction and sintering at 650°C for 2 h, the green body of the CuO-SnO2 is completely converted into porous Cu-Sn alloy. Microstructural observation reveals that the sintered samples have large pores which are aligned parallel to the camphene growth direction. The size of the large pores increases from 150 to 300 μm with an increase in the holding time. Also, the internal walls of the large pores contain relatively small pores whose size increases with the holding time. The change in pore structure is explained by the growth behavior of the camphene crystals and rearrangement of the solid particles during the freezing process.
-
Citations
Citations to this article as recorded by- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
Jae-Hun Jeong, Sung-Tag Oh, Chang-Yong Hyun
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 6. CrossRef - Fabrication of Al2O3 Dispersed Porous Cu by Freeze Drying of CuO-Al2O3/Camphene Slurry
Hyunji Kang, Doh-Hyung Riu, Sung-Tag Oh
journal of Korean Powder Metallurgy Institute.2018; 25(1): 25. CrossRef - Porous W-Ni Alloys Synthesized from Camphene/WO3-NiO Slurry by Freeze Drying and Heat Treatment in Hydrogen Atmosphere
Sung Hyun Park, Seong-Min Park, So-Jeong Park, Bo-Yeong Park, Sung-Tag Oh
Korean Journal of Materials Research.2018; 28(2): 108. CrossRef
- Fabrication of Porous Ni by Freeze Drying and Hydrogen Reduction of NiO/Camphene Slurry
- [Korean]
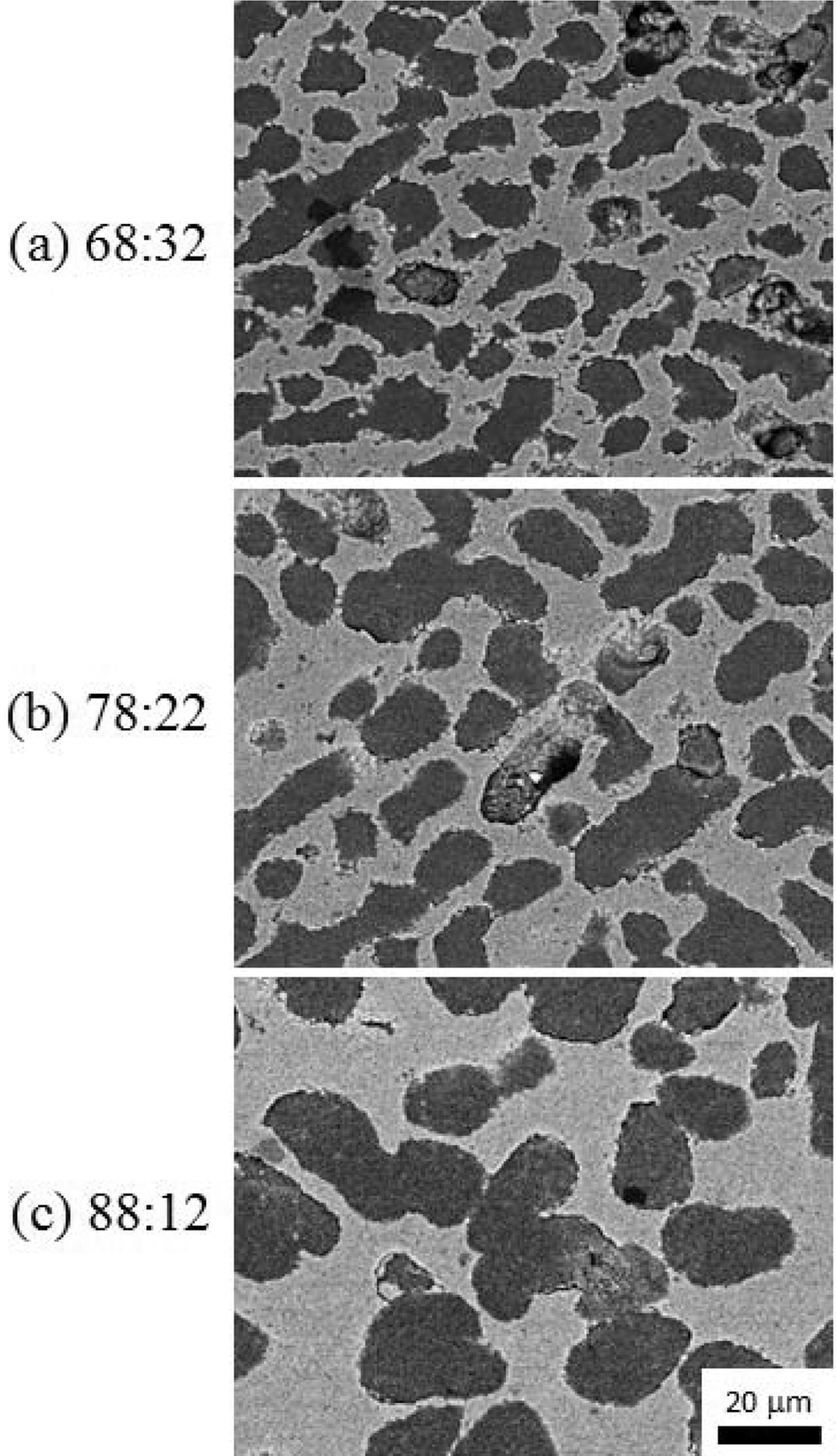
- Fabrication of Porous Al2O3 Film by Freeze Tape Casting
- Ran-Hee Shin, Jun-Mo Koo, Young-Do Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2015;22(6):438-442. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.438
- 513 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF Porous thick film of alumina which is fabricated by freeze tape casting using a camphene-camphor-acrylate vehicle. Alumina slurry is mixed above the melting point of the camphene-camphor solvent. Upon cooling, the camphene-camphor crystallizes from the solution as particle-free dendrites, with the Al2O3 powder and acrylate liquid in the interdendritic spaces. Subsequently, the acrylate liquid is solidified by photopolymerization to offer mechanical properties for handling. The microstructure of the porous alumina film is characterized for systems with different cooling rate around the melting temperature of camphor-camphene. The structure of the dendritic porosity is compared as a function of ratio of camphene-camphor solvent and acrylate content, and Al2O3 powder volume fraction in acrylate in terms of the dendrite arm width.
- [Korean]
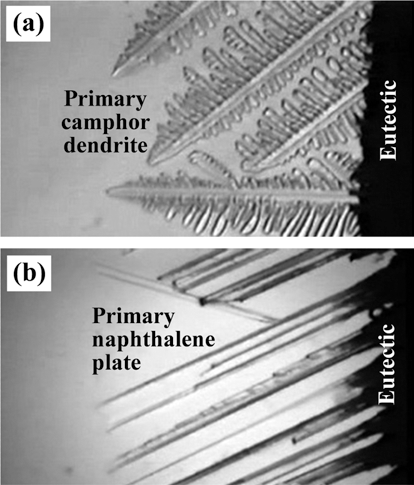
- Effect of Sublimable Vehicle Compositions in the Camphor-Naphthalene System on the Pore Structure of Porous Cu-Ni
- Na-Yeon Kwon, Myung-Jin Suka, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(5):362-366. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.362
- 800 View
- 1 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The effect of sublimable vehicle composition in the camphor-naphthalene system on the pore structure of porous Cu-Ni alloy is investigated. The CuO-NiO mixed slurries with hypoeutectic, eutectic and hypereutectic compositions are frozen into a mold at -25°C. Pores are generated by sublimation of the vehicles at room temperature. After hydrogen reduction at 300°C and sintering at 850°C for 1 h, the green body of CuO-NiO is completely converted to porous Cu-Ni alloy with various pore structures. The sintered samples show large pores which are aligned parallel to the sublimable vehicle growth direction. The pore size and porosity decrease with increase in powder content due to the degree of powder rearrangement in slurry. In the hypoeutectic composition slurry, small pores with dendritic morphology are observed in the sintered Cu-Ni, whereas the specimen of hypereutectic composition shows pore structure of plate shape. The change of pore structure is explained by growth behavior of primary camphor and naphthalene crystals during solidification of camphor-naphthalene alloys.
-
Citations
Citations to this article as recorded by- Freeze Drying Process and Pore Structure Characteristics of Porous Cu with Various Sublimable Vehicles
Gyuhwi Lee, Sung-Tag Oh, Myung-Jin Suk, Young-Keun Jeong
Journal of Korean Powder Metallurgy Institute.2020; 27(3): 198. CrossRef - Interaction of Solid Particles with the Solidifying Front in the Liquid-Particle Mixture
Ho-Suk Lee, Kyu-Hee Lee, Sung-Tag Oh, Young Do Kim, Myung-Jin Suk
Journal of Korean Powder Metallurgy Institute.2018; 25(4): 336. CrossRef
- Freeze Drying Process and Pore Structure Characteristics of Porous Cu with Various Sublimable Vehicles
- [Korean]
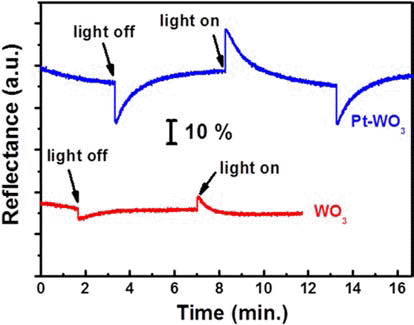
- Fabrication of Photoelectrochromic Devices Composed of Anodized TiO2 and WO3 Nanostructures
- Sanghoon Lee, Hyeongcheol Cha, Yoon-Chae Nah
- J Korean Powder Metall Inst. 2015;22(5):326-330. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.326
- 515 View
- 3 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, we demonstrate the photoelectrochromic devices composed of TiO2 and WO3 nanostructures prepared by anodization method. The morphology and the crystal structure of anodized TiO2 nanotubes and WO3 nanoporous layers are investigated by SEM and XRD. To fabricate a transparent photoelectrode on FTO substrate, a TiO2 nanotube membrane, which has been detached from Ti substrate, is transferred to FTO substrate and annealed at 450°C for 1 hr. The photoelectrode of TiO2 nanotube and the counter electrode of WO3 nanoporous layer are assembled and the inner space is filled with a liquid electrolyte containing 0.5 M LiI and 5 mM I2 as a redox mediator. The properties of the photoelectrochromic devices is investigated and Pt-WO3 electrode system shows better electrochromic performance compared toWO3 electrode.
-
Citations
Citations to this article as recorded by- Synthesis and characterization of nitrogen-doped TiO 2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity
Yifan Zhang, Hye Mee Yang, Soo-Jin Park
Current Applied Physics.2018; 18(2): 163. CrossRef - Photocatalytic and Adsorption Properties of WO3 Nanorods Prepared by Hydrothermal Synthesis
Su-Yeol Yu, Chunghee Nam
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 483. CrossRef - Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
Hyeong-Chul Kim, Jae-Kil Han
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 33. CrossRef
- Synthesis and characterization of nitrogen-doped TiO 2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity
- [Korean]
- Formation of Nano-oxides on Porous Metallic Glass Compacts using Hydrothermal Synthesis
- H. J. Park, Y. S. Kim, S. H. Hong, J. T. Kim, J. Y. Cho, W. H. Lee, K. B. Kim
- J Korean Powder Metall Inst. 2015;22(4):229-233. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.229

- 594 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous metallic glass compact (PMGC) are developed by electro-discharge sintering (EDS) process of gas atomized Zr41.2Ti13.8Cu12.5Ni10Be22.5 metallic glass powder under of 0.2 kJ generated by a 450 μF capacitor being charged to 0.94 kV. Functional iron-oxides are formed and growth on the surface of PMGCs via hydrothermal synthesis. It is carried out at 150°C for 48hr with distilled water of 100 mL containing Fe ions of 0.18 g/L. Consequently, two types of iron oxides with different morphology which are disc-shaped Fe2O3 and needle-shaped Fe3O4 are successfully formed on the surface of the PMGCs. This finding suggests that PMGC witih hydrothermal technique can be attractive for the practical technology as a new area of structural and functional materials. And they provide a promising road map for using the metallic glasses as a potential functional application.
-
Citations
Citations to this article as recorded by- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
- [Korean]
- Fabrication and Mechanical Properties of STS316L Porous Metal for Vacuum Injection Mold
- Se Hoon Kim, Sang Min Kim, Sang Ho Noh, Jin Pyeong Kim, Jae Hyuck Shin, Si-Young Sung, Jin Kwang Jin, Taean Kim
- J Korean Powder Metall Inst. 2015;22(3):197-202. Published online June 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.3.197
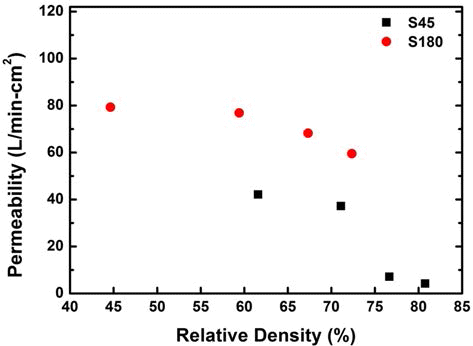
- 665 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, porous stainless steel (STS316L) sintered body was fabricated by powder metallurgy method and its properties such as porosity, compressive yield strength, hardness, and permeability were evaluated. 67.5Fe-17Cr- 13Ni-2.5Mo (wt%) powder was produced by a water atomization. The atomized powder was classified into size with under 45 μm and over 180 μm, and then they were compacted with various pressures and sintered at 1210°C for 1 h in a vacuum atmosphere. The porosities of sintered bodies could be obtained in range of 20~53% by controlling the compaction pressure. Compressive yield strength and hardness were achieved up to 268 MPa and 94 Shore D, respectively. Air permeability was obtained up to 79 l/min·cm2. As a result, mechanical properties and air permeability of the optimized porous body having a porosity of 25~40% were very superior to that of Al alloy.
-
Citations
Citations to this article as recorded by- Printability and physical properties of iron slag powder composites using material extrusion-based 3D printing
Hyungjin Kim, Sangkyu Lee
Journal of Iron and Steel Research International.2021; 28(1): 111. CrossRef - Study on Effects of Mold Temperature on the Injection Molded Article
J.-H. Han, Y.-C. Kim
Archives of Metallurgy and Materials.2017; 62(2): 1271. CrossRef
- Printability and physical properties of iron slag powder composites using material extrusion-based 3D printing
- [Korean]
- Microstructure and Tensile Deformation Behavior of Ni-Cr-Al Powder Porous Block Material
- Chul-O Kim, Jung-Suk Bae, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2015;22(2):93-99. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.93
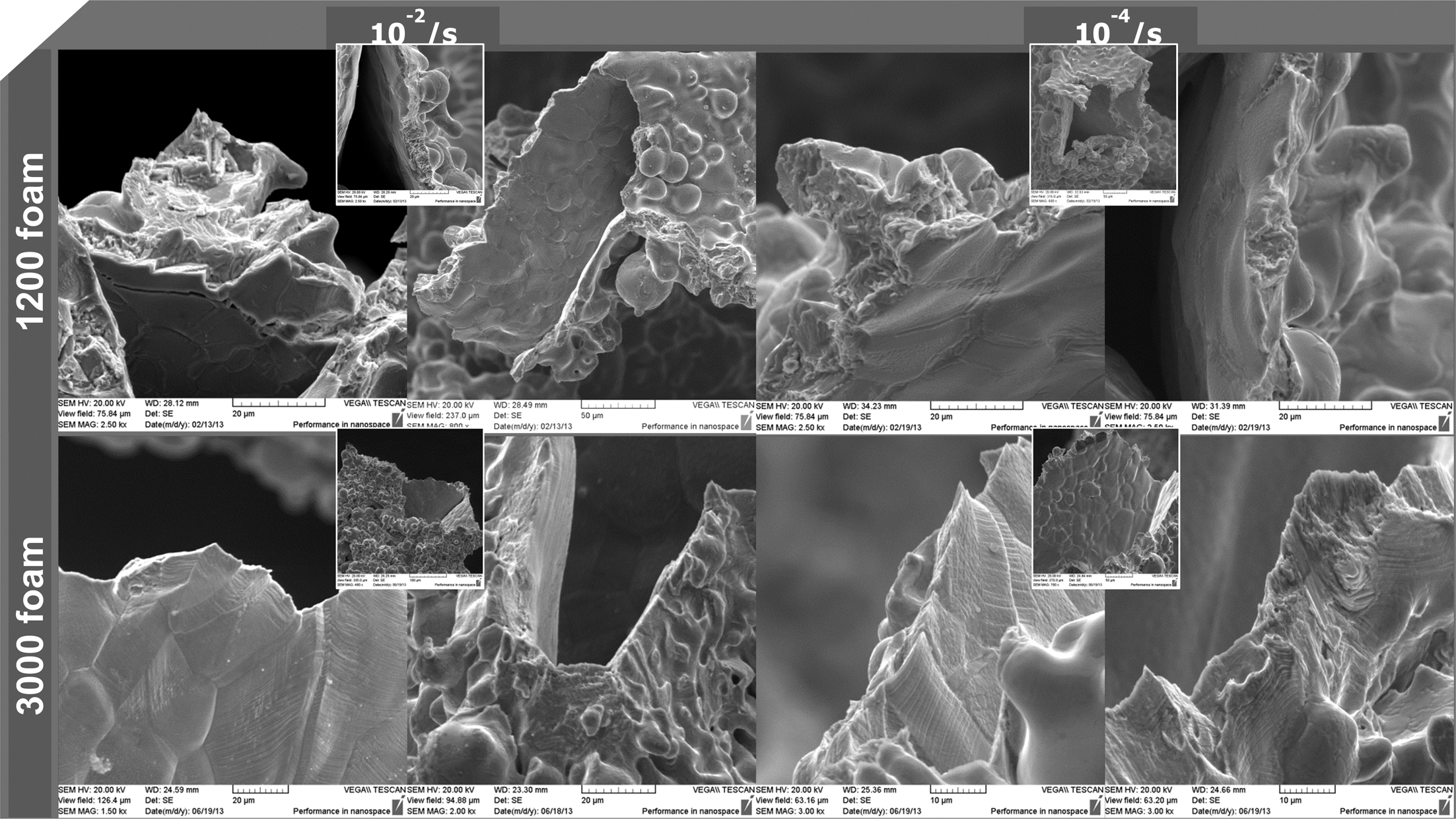
- 560 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF This study investigated the microstructure and tensile properties of a recently made block-type Ni-Cr-Al powder porous material. The block-type powder porous material was made by stacking multiple layers of powder porous thin plates with post-processing such as additional compression and sintering. This study used block-type powder porous materials with two different cell sizes: one with an average cell size of 1,200 μm (1200 foam) and the other with an average cell size of 3,000 μm (3000 foam). The γ-Ni and γ’-Ni3Al were identified as the main phases of both materials. However, in the case of the 1,200 foam, a β-NiAl phase was additionally observed. The relative density of each block-type powder porous material, with 1200 foam and 3000 foam, was measured to be 5.78% and 2.93%, respectively. Tensile tests were conducted with strain rates of 10−2~10−4 sec−1. The test result showed that the tensile strength of the 1,200 foam was 6.0~7.1 MPa, and that of 3,000 foam was 3.0~3.3 MPa. The elongation of the 3,000 foam was higher (~9%) than that (~2%) of the 1,200 foam. This study also discussed the deformation behavior of block-type powder porous material through observations of the fracture surface, with the results above.
-
Citations
Citations to this article as recorded by- Effect of Strut Thickness on Room and High Temperature Compressive Properties of Block-Type Ni-Cr-Al Powder Porous Metals
B.-H. Kang, M.-H. Park, K.-A. Lee
Archives of Metallurgy and Materials.2017; 62(2): 1329. CrossRef - Fabrication and Shape Memory Characteristics of Highly Porous Ti-Nb-Mo Biomaterials
Y.-W. Kim, T.W. Mukarati
Archives of Metallurgy and Materials.2017; 62(2): 1367. CrossRef
- Effect of Strut Thickness on Room and High Temperature Compressive Properties of Block-Type Ni-Cr-Al Powder Porous Metals
- [Korean]
- Fabrication of Porous W by Heat Treatment of Pore Forming Agent of PMMA and WO3 Powder Compacts
- Ki Cheol Jeon, Young Do Kim, Myung-Jin Suk, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(2):129-133. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.129
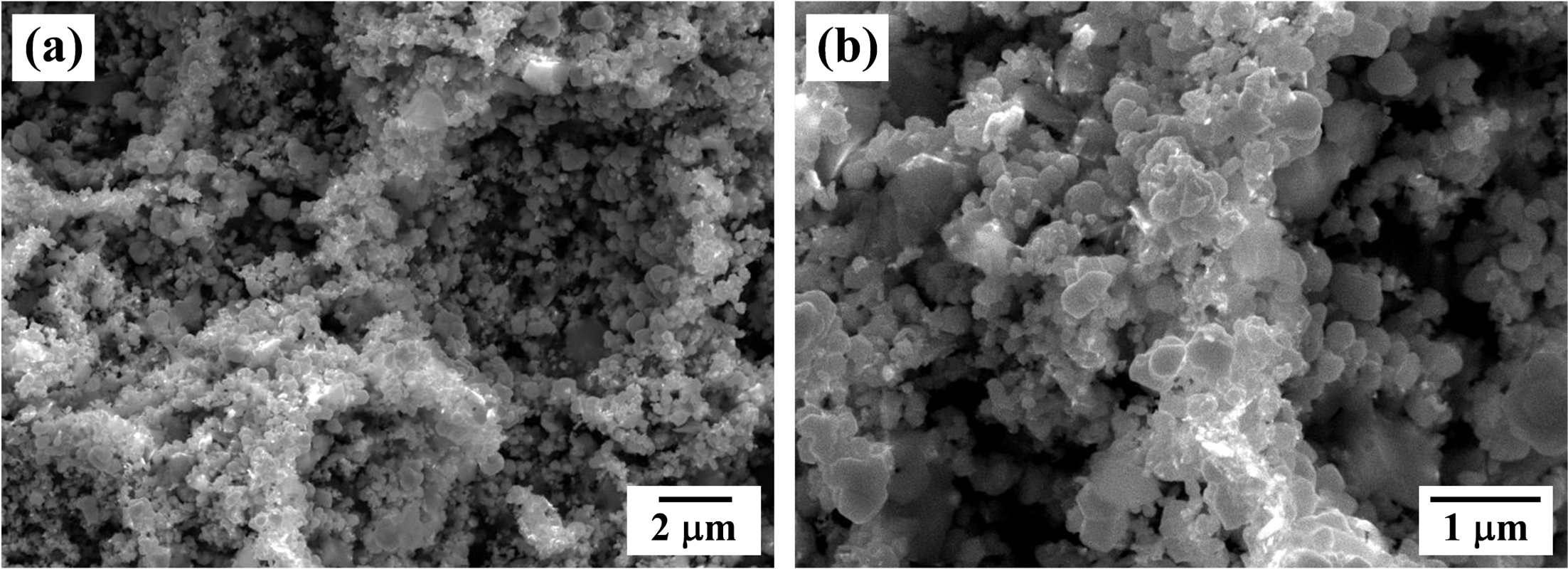
- 1,246 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Porous W with controlled pore structure was fabricated by thermal decomposition and hydrogen reduction process of PMMA beads and WO3 powder compacts. The PMMA sizes of 8 and 50 μm were used as pore forming agent for fabricating the porous W. The WO3 powder compacts with 20 and 70 vol% PMMA were prepared by uniaxial pressing and sintered for 2 h at 1200°C in hydrogen atmosphere. TGA analysis revealed that the PMMA was decomposed at about 400°C and WO3 was reduced to metallic W at 800°C. Large pores in the sintered specimens were formed by thermal decomposition of spherical PMMA, and their size was increased with increase in PMMA size and the amount of PMMA addition. Also the pore shape was changed from spherical to irregular form with increasing PMMA contents due to the agglomeration of PMMA in the powder mixing process.
-
Citations
Citations to this article as recorded by- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
Young-Sang Cho, Nahee Ku, Young-Seok Kim
JOURNAL OF CHEMICAL ENGINEERING OF JAPAN.2019; 52(2): 194. CrossRef
- Synthesis of Porous Silica Particles Using Sodium Silicate Precursor for Water-Repellent Surfaces
- [Korean]
- Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
- Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(2):122-128. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.122
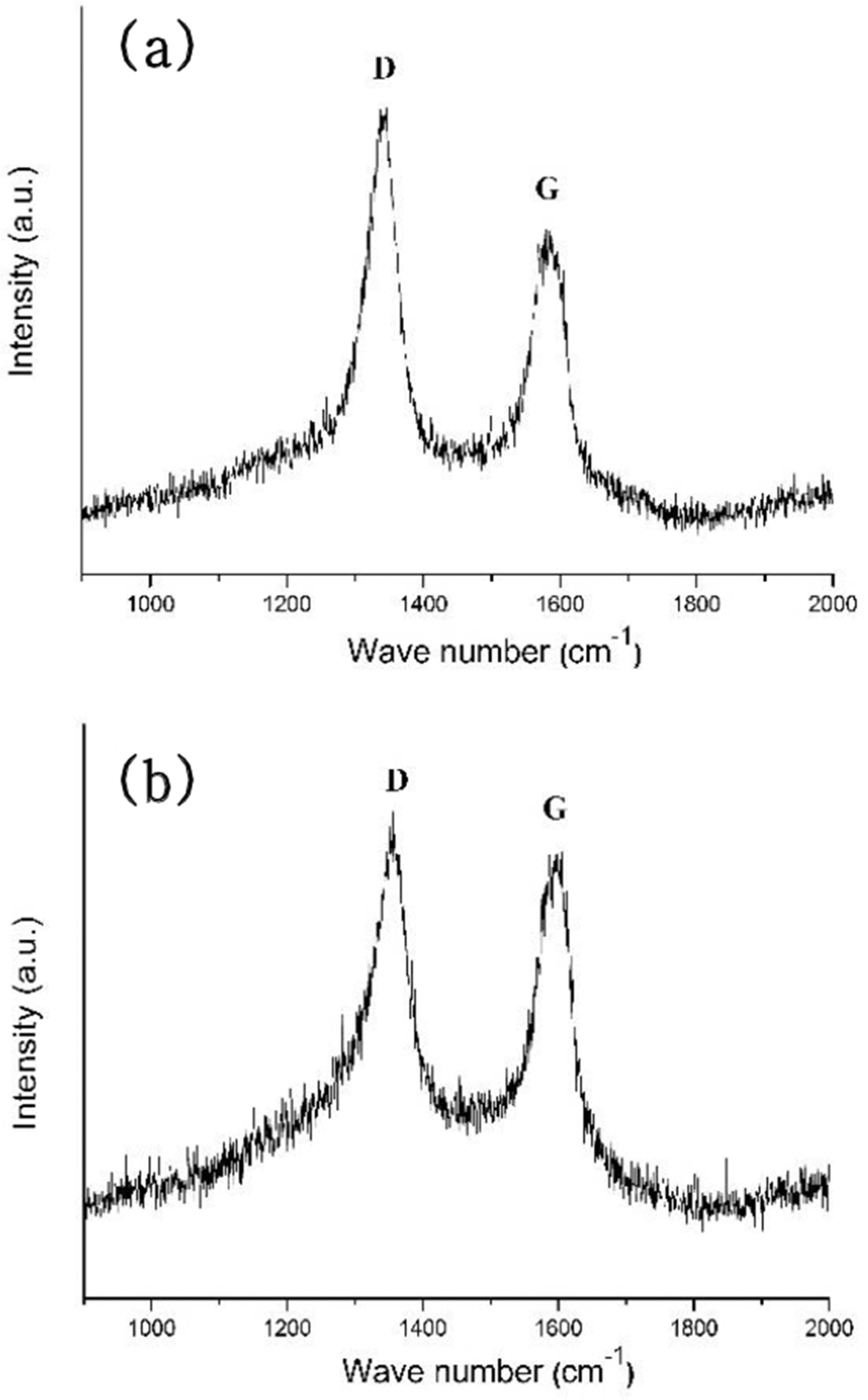
- 582 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, titanium(Ti) meshes and porous bodies are employed to synthesize carbon nanotubes(CNTs) using methane(CH4) gas and camphene solution, respectively, by chemical vapor deposition. Camphene is impregnated into Ti porous bodies prior to heating in a furnace. Various microscopic and spectroscopic techniques are utilized to analyze CNTs. It is found that CNTs are more densely and homogeneously populated on the camphene impregnated Ti-porous bodies as compared to CNTs synthesized with methane on Ti-porous bodies. It is elucidated that, when synthesized with methane, few CNTs are formed inside of Ti porous bodies due to methane supply limited by internal structures of Ti porous bodies. Ti-meshes and porous bodies are found to be multi-walled with high degree of structural disorders. These CNTs are expected to be utilized as catalyst supports in catalytic filters and purification systems.
-
Citations
Citations to this article as recorded by- Recent progress in additive manufacturing of porous titanium: From design to applications
Haoxin Song, Chen Wang, Wenzheng Yu, Mingsen Zhang, Jinqiang Shao, Hanwen Liang, Tingting Wu, Xiaoxiao Dong
Journal of Alloys and Compounds.2025; 1026: 180451. CrossRef - Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef
- Recent progress in additive manufacturing of porous titanium: From design to applications
- [Korean]
- Fabrication of Fe-Cr-Al Porous Metal with Sintering Temperature and Times
- Bon-Uk Koo, Su-in Lee, Dahee Park, Jung-Yeul Yun, Byoung-Kee Kim
- J Korean Powder Metall Inst. 2015;22(2):100-104. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.100

- 583 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The porous metals are known as relatively excellent characteristic such as large surface area, light, lower heat capacity, high toughness and permeability. The Fe-Cr-Al alloys have high corrosion resistance, heat resistance and chemical stability for high temperature applications. And then many researches are developed the Fe-Cr-Al porous metals for exhaust gas filter, hydrogen reformer catalyst support and chemical filter. In this study, the Fe-Cr-Al porous metals are developed with Fe-22Cr-6Al(wt) powder using powder compaction method. The mean size of Fe-22Cr-6Al(wt) powders is about 42.69 μm. In order to control pore size and porosity, Fe-Cr-Al powders are sintered at 1200~1450°C and different sintering maintenance as 1~4 hours. The powders are pressed on disk shapes of 3 mm thickness using uniaxial press machine and sintered in high vacuum condition. The pore properties are evaluated using capillary flow porometer. As sintering temperature increased, relative density is increased from 73% to 96% and porosity, pore size are decreased from 27 to 3.3%, from 3.1 to 1.8 μm respectively. When the sintering time is increased, the relative density is also increased from 76.5% to 84.7% and porosity, pore size are decreased from 23.5% to 15.3%, from 2.7 to 2.08 μm respectively.
-
Citations
Citations to this article as recorded by- Synthesis and Magnetic Hysteresis Properties of an Aluminum-Doped Isotropic Hard-Magnetic Fe–Cr–Co Powder Alloy
A. S. Ustyukhin, V. A. Zelensky, I. M. Milyaev, M. I. Alymov, A. A. Ashmarin, A. B. Ankudinov, K. V. Sergienko
Inorganic Materials: Applied Research.2024; 15(2): 480. CrossRef - Fabrication of a Porous Ni-Based Metal with a Multi-pore Structure by a Screen Printing Process
Yu-Jeong Yi, Min-Jeong Lee, Jung-Yeul Yun, Byoung-Kee Kim
Metals and Materials International.2019; 25(5): 1272. CrossRef
- Synthesis and Magnetic Hysteresis Properties of an Aluminum-Doped Isotropic Hard-Magnetic Fe–Cr–Co Powder Alloy
- [English]
- Preparation and Characterization of Porous Silicon and Carbon Composite as an Anode Material for Lithium Rechargeable Batteries
- Junsoo Park, Jae-won Lee
- J Korean Powder Metall Inst. 2015;22(1):15-20. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.15
- 583 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The composite of porous silicon (Si) and amorphous carbon (C) is prepared by pyrolysis of a nano-porous Si + pitch mixture. The nano-porous Si is prepared by mechanical milling of magnesium powder with silicon monoxide (SiO) followed by removal of MgO with hydrochloric acid (etching process). The Brunauer-Emmett-Teller (BET) surface area of porous Si (64.52 m2g−1) is much higher than that before etching Si/MgO (4.28 m2g−1) which indicates pores are formed in Si after the etching process. Cycling stability is examined for the nano-porous Si + C composite and the result is compared with the composite of nonporous Si + C. The capacity retention of the former composite is 59.6% after 50 charge/discharge cycles while the latter shows only 28.0%. The pores of Si formed after the etching process is believed to accommodate large volumetric change of Si during charging and discharging process.
- [English]
- Processing Methods for the Preparation of Porous Ceramics
- Rizwan Ahmad, Jang-Hoon Ha, In-Hyuck Song
- J Korean Powder Metall Inst. 2014;21(5):389-398. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.389
- 3,030 View
- 95 Download
- 22 Citations
-
 Abstract
Abstract
 PDF
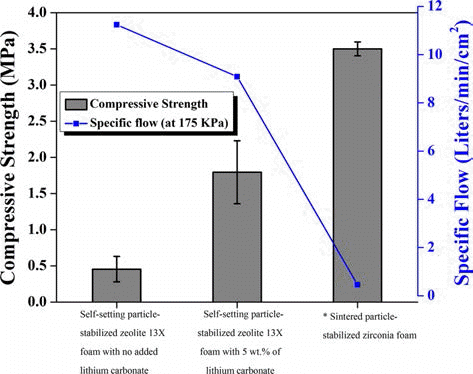
PDF Macroporous ceramics with tailored pore size and shape could be used for well-established and emerging applications, such as molten metal filtration, biomaterial, catalysis, thermal insulation, hot gas filtration and diesel particulate filters. In these applications, unique properties of porous materials were required which could be achieved through the incorporation of macro-pores into ceramics. In this article, we reviewed the main processing techniques which can be used for the fabrication of macroporous ceramics with tailored microstructure. Partial sintering, replica templates, sacrificial fugutives, and direct foaming techniques was described here and compared in terms of micro-structures and mechanical properties that could be achieved. The main focus was given to the direct foaming technique which was simple and versatile approach that allowed the fabrication of macro-porous ceramics with tailored features and properties.
-
Citations
Citations to this article as recorded by- Contribution of microscale stochastic truss models to investigate the macroscale elasticity constants of porous ceramics
Thierry Canet, Gilles Dusserre, Thierry Cutard
European Journal of Mechanics - A/Solids.2025; 111: 105561. CrossRef - Synthesis and properties of high alumina cement-based porous composites in the ZrO2-CaO-Al2O3 system
Yesica L. Bruni, María F. Hernández, Susana Conconi, Gustavo Suárez
Ceramics International.2025; 51(23): 39794. CrossRef - Morphology and phase analysis of cordierite ceramic foams with Ag nanoparticles
J. Kupková, G. Kratošová, K. Čech Barabaszová, G. Simha Martynková
IOP Conference Series: Materials Science and Engineering.2025; 1337(1): 012004. CrossRef - Organic waste-derived pore formers for macroporous ceramics fabrication: A review on synthesis, durability properties and potential applications
T.T. Dele-Afolabi, M.A. Azmah Hanim, A.A. Oyekanmi, M.N.M. Ansari, Surajudeen Sikiru, O.J. Ojo-Kupoluyi
Materials Today Sustainability.2024; 27: 100824. CrossRef - Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review
Kamrun Nahar Fatema, Md Rokon Ud Dowla Biswas, Jung Gyu Park, Ik Jin Kim
Micro.2024; 4(4): 552. CrossRef - Investigating mass transfer coefficients in lean methane combustion reaction through the morphological and geometric analysis of structured open cell foam catalysts
Carmen W. Moncada Quintero, Hernan G. Mazzei, Marion Servel, Frédéric Augier, Yacine Haroun, Jean-François Joly, Stefania Specchia
Chemical Engineering Science.2023; 281: 119138. CrossRef - Composite PLGA–Nanobioceramic Coating on Moxifloxacin-Loaded Akermanite 3D Porous Scaffolds for Bone Tissue Regeneration
Georgia K. Pouroutzidou, Lambrini Papadopoulou, Maria Lazaridou, Konstantinos Tsachouridis, Chrysanthi Papoulia, Dimitra Patsiaoura, Ioannis Tsamesidis, Konstantinos Chrissafis, George Vourlias, Konstantinos M. Paraskevopoulos, Antonios D. Anastasiou, Dim
Pharmaceutics.2023; 15(3): 819. CrossRef - Development of high strength large open porosity alumina ceramics using the sacrificial phase route: The role of the sacrificial phase fineness
Julian Alzukaimi, Rafi Jabrah
Ceramics International.2023; 49(2): 2923. CrossRef - Sustainable nanocomposite porous absorbent and membrane sieves: Definition, classification, history, properties, synthesis, applications, and future prospects
Sameer Ahmad, Weqar Ahmad Siddiqi, Sharif Ahmad
Journal of Environmental Chemical Engineering.2023; 11(2): 109367. CrossRef - Processing and characterization of porous composites based on CaAl4O7/CaZrO3
Yesica L. Bruni, María S. Conconi, María F. Hernández, Gustavo Suárez
Ceramics International.2023; 49(23): 37630. CrossRef - Trace addition of cellulose nanofiber in gel-casting system for structurally controlled porous ceramics towards superior thermal-insulating property
Yunzi Xin, Takashi Shirai
Journal of the Ceramic Society of Japan.2022; 130(5): 355. CrossRef - State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
Ismail Barbaros, Yongmin Yang, Babak Safaei, Zhicheng Yang, Zhaoye Qin, Mohammed Asmael
Nanotechnology Reviews.2022; 11(1): 321. CrossRef - A review of petroleum emulsification types, formation factors, and demulsification methods
Ahmed Abdulrazzaq Hadi, Ali Abdulkhabeer Ali
Materials Today: Proceedings.2022; 53: 273. CrossRef - SLURRY OPTIMISATION FOR FAST FREEZE-DRYING OF POROUS ALUMINA
Kritkaew Somton
Ceramics - Silikaty.2021; : 368. CrossRef - Recycling food, agricultural, and industrial wastes as pore-forming agents for sustainable porous ceramic production: A review
Siti Zuliana Salleh, Afiqah Awang Kechik, Abdul Hafidz Yusoff, Mustaffa Ali Azhar Taib, Maryana Mohamad Nor, Mardawani Mohamad, Tse Guan Tan, Arlina Ali, Mohamad Najmi Masri, Julie Juliewatty Mohamed, Siti Koriah Zakaria, Jia Geng Boon, Faisal Budiman, Pa
Journal of Cleaner Production.2021; 306: 127264. CrossRef - Novel noble-metal-free ceramic filter with controlled pore structure for environmental cleaning
Yunzi Xin, Sohei Nakagawa, Harumitsu Nishikawa, Takashi Shirai
Ceramics International.2021; 47(8): 11819. CrossRef - Fabrication of porous titania sheet via tape casting: Microstructure and water permeability study
Saber Ghannadi, Hossein Abdizadeh, Alireza Babaei
Ceramics International.2020; 46(7): 8689. CrossRef - Farklı Bağlayıcı ve Sinterleme Katkılarının SiC Seramik Prefom Mikroyapısı Üzerine Etkisi
Ebru Yılmaz, Fatih Çalışkan
Academic Perspective Procedia.2019; 2(3): 1309. CrossRef - The preparation and characterization of porous alumina ceramics using an eco‐friendly pore‐forming agent
Julian Alzukaimi, Rafi Jabrah
International Journal of Applied Ceramic Technology.2019; 16(2): 820. CrossRef - Wet Foam Stability from Colloidal Suspension to Porous Ceramics: A Review
Ik Jin Kim, Jung Gyu Park, Young Han Han, Suk Young Kim, James F. Shackelford
Journal of the Korean Ceramic Society.2019; 56(3): 211. CrossRef - Processing of porous alumina by foaming method-effect of foaming agent, solid loading and binder
Soumya Devavarapu, Paritosh Chaudhuri, Aroh Shrivastava, Santanu Bhattacharyya
Ceramics International.2019; 45(9): 12264. CrossRef - Macroporous flexible polyvinyl alcohol lithium adsorbent foam composite prepared via surfactant blending and cryo-desiccation
Grace M. Nisola, Lawrence A. Limjuco, Eleazer L. Vivas, Chosel P. Lawagon, Myoung Jun Park, Ho Kyong Shon, Neha Mittal, In Wook Nah, Hern Kim, Wook-Jin Chung
Chemical Engineering Journal.2015; 280: 536. CrossRef
- Contribution of microscale stochastic truss models to investigate the macroscale elasticity constants of porous ceramics
- [English]
- Production of Porous Metallic Glass Granule by Optimizing Chemical Processing
- Song-Yi Kim, Bo-Kyung Guem, Min-Ha Lee, Taek-Soo Kim, Jurgen Eckert, Bum-Sung Kim
- J Korean Powder Metall Inst. 2014;21(4):251-255. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.251
- 878 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
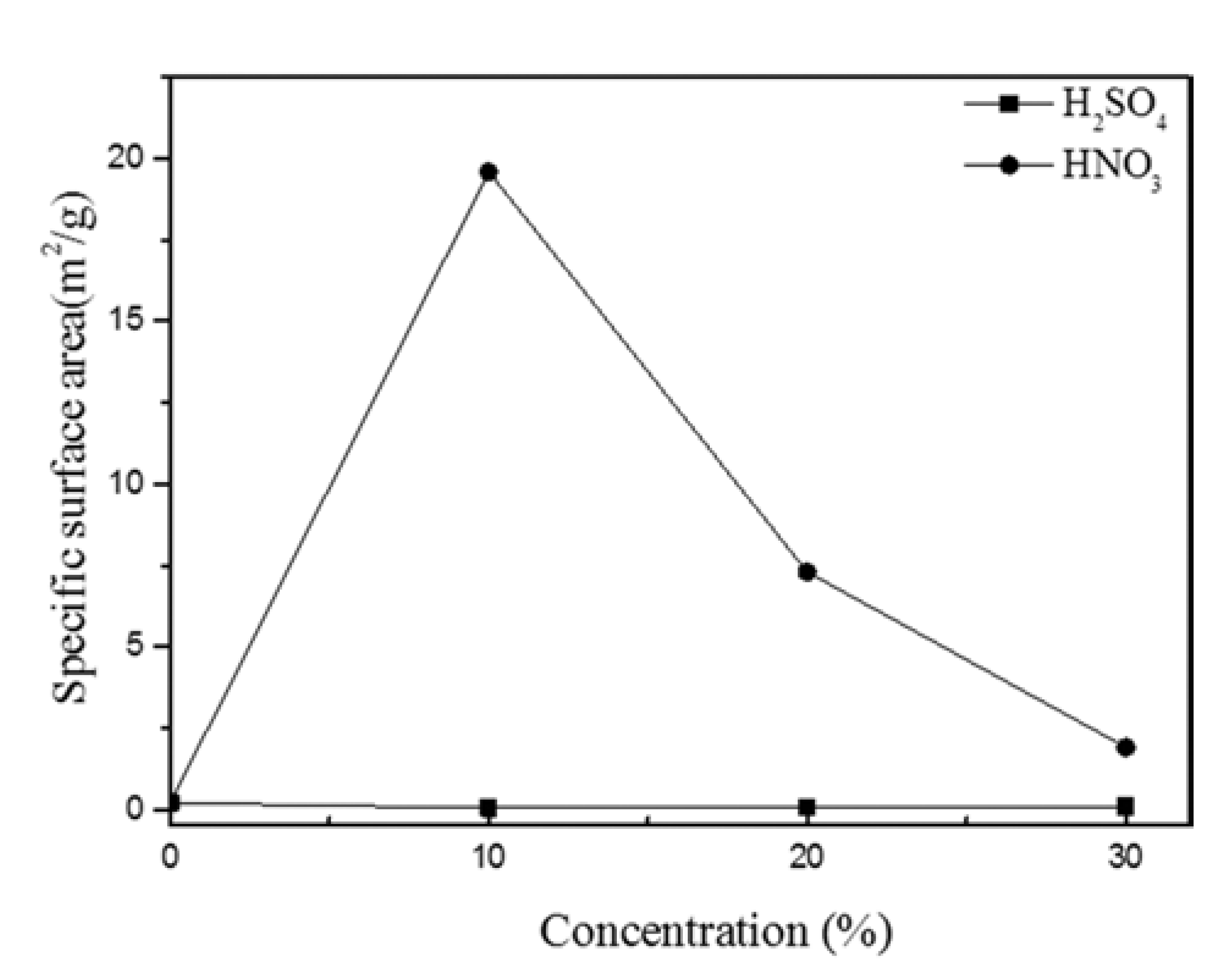
PDF In this study, we optimized dissolution the dissolution conditions of porous amorphous powder to have high specific surface area. Porous metallic glass(MG) granules were fabricated by selective phase dissolution, in which brass is removed from a composite powder consisting of MG and 40 vol.% brass. Dissolution was achieved through various concentrations of H2SO4 and HNO3, with HNO3 proving to have the faster reaction kinetics. Porous powders were analyzed by differential scanning calorimetry to observe crystallization behavior. The Microstructure of milled powder and dissolved powder was analyzed by scanning electron microscope. To check for residual in the dissolved powder after dissolution, energy dispersive X-ray spectroscory and elemental mapping was conducted. It was confirmed that the MG/brass composite powder dissolved in 10% HNO3 produced a porous MG granule with a relatively high specific surface area of 19.60 m2/g. This proved to be the optimum dissolution condition in which both a porous internal granule structure and amorphous phase were maintained. Consequently, porous MG granules were effectively fabricated and applications of such structures can be expanded.
-
Citations
Citations to this article as recorded by- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
- [Korean]
- Fabrication of Porous Cu by Freeze-drying Process of Camphene Slurry with CuO-coated Cu Powders
- Su-Ryong Bang, Sung-Tag Oh
- J Korean Powder Metall Inst. 2014;21(3):191-195. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.191
- 635 View
- 0 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
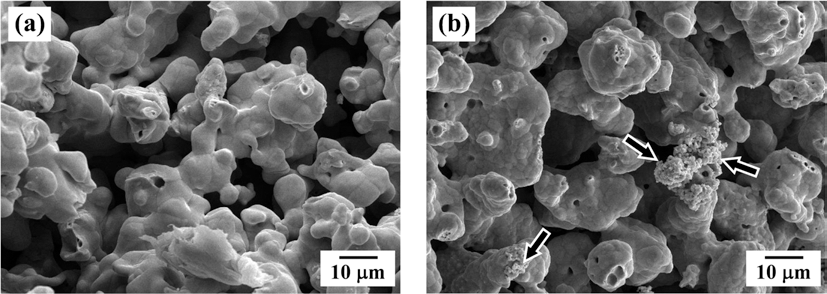
PDF This study reports a simple way of fabricating the porous Cu with unidirectional pore channels by freeze drying camphene slurry with Cu oxide coated Cu powders. The coated powders were prepared by calcination of ballmilled powder mixture of Cu and Cu-nitrate. Improved dispersion stability of camphene slurry could be achieved using the Cu oxide coated Cu powders instead of pure Cu powders. Pores in the frozen specimen at -25°C were generated by sublimation of the camphene during drying in air, and the green bodies were sintered at 750°C for 1 h in H2 atmosphere. XRD analysis revealed that the coated layer of Cu oxide was completely converted to Cu phase without any reaction phases by hydrogen heat treatment. The porous Cu specimen prepared from pure Cu powders showed partly large pores with unidirectional pore channels, but most of pores were randomly distributed. In contrast, large and aligned parallel pores to the camphene growth direction were clearly observed in the sample using Cu oxide coated Cu powders. Pore formation behavior depending on the initial powders was discussed based on the degree of powder rearrangement and dispersion stability in slurry.
-
Citations
Citations to this article as recorded by- Effect of Freezing and Sintering Condition of CuO-SnO2/Camphene Slurries on the Pore Structure of Porous Cu-Sn
Joo-Hyung Kim, Sung-Tag Oh, Chang-Yong Hyun
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 49. CrossRef - Fabrication of Cu-30 vol% SiC Composites by Pressureless Sintering of Polycarbosilane Coated SiC and Cu Powder Mixtures
Yeon Su Kim, Na-Yeon Kwon, Young-Keun Jeong, Sung-Tag Oh
Korean Journal of Materials Research.2016; 26(6): 337. CrossRef - Synthesis of Aligned Porous Sn by Freeze-Drying of Tin Chloride/camphene Slurry
수룡 방, 승탁 오
Korean Journal of Materials Research.2015; 25(1): 27~31. CrossRef
- Effect of Freezing and Sintering Condition of CuO-SnO2/Camphene Slurries on the Pore Structure of Porous Cu-Sn
- [Korean]
- Effect of Cell Size on the High Temperature Oxidation Properties of Fe-Cr-Al Powder Porous Metal Manufactured by Electro-spray Process
- Jae-Sung Oh, Young-Min Kong, Byoung-Kee Kim, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2014;21(1):55-61. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.55

- 802 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Fe-Cr-Al powder porous metal was manufactured by using new electro-spray process. First, ultra-fine fecralloy powders were produced by using the submerged electric wire explosion process. Evenly distributed colloid (0.05~0.5% powders) was dispersed on Polyurethane foam through the electro-spray process. And then degreasing and sintering processes were conduced. In order to examine the effect of cell size (200 μm, 450 μm, 500 μm) in process, pre-samples were sintered for two hours at temperature of 1450°C, in H2 atmospheres. A 24-hour thermo gravimetric analysis test was conducted at 1000°C in a 79% N2 + 21% O2 to investigate the high temperature oxidation behavior of powder porous metal. The results of the high temperature oxidation tests showed that oxidation resistance increased with increasing cell size. In the 200 μm porous metal with a thinner strut and larger specific surface area, the depletion of the stabilizing elements such as Al and Cr occurred more quickly during the high-temperature oxidation compared with the 450, 500 μm porous metals.
-
Citations
Citations to this article as recorded by- Fabrication and Mechanical Properties of Open‐Cell Austenitic Stainless Steel Foam by Electrostatic Powder Spraying Process
Tae-Hoon Kang, Kyu-Sik Kim, Jung-Yeul Yun, Min-Jeong Lee, Kee-Ahn Lee
Advanced Engineering Materials.2020;[Epub] CrossRef - Microstructure and High Temperature Oxidation Behaviors of Fe-Ni Alloys by Spark Plasma Sintering
Chae Hong Lim, Jong Seok Park, Sangsun Yang, Jung-Yeul Yun, Jin Kyu Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(1): 53. CrossRef - Effect of Al2O3 Inter-Layer Grown on FeCrAl Alloy Foam to Improve the Dispersion and Stability of NiO Catalysts
유진 이, 본율 구, 성호 백, 만호 박, 효진 안
Korean Journal of Materials Research.2015; 25(8): 391~397. CrossRef
- Fabrication and Mechanical Properties of Open‐Cell Austenitic Stainless Steel Foam by Electrostatic Powder Spraying Process
- [Korean]
- Fabrication of Porous Cu-Ni by Freeze Drying and Hydrogen Reduction of CuO-NiO Powder Mixture
- Han Gil Seo, Sung-Tag Oh
- J Korean Powder Metall Inst. 2014;21(1):34-38. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.34

- 691 View
- 0 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Cu-Ni alloys with unidirectionally aligned pores were prepared by freeze-drying process of CuO-NiO/camphene slurry. Camphene slurries with dispersion stability by the addition of oligomeric polyester were frozen at -25°C, and pores in the frozen specimens were generated by sublimation of the camphene during drying in air. The green bodies were hydrogen-reduced at 300°C and sintered at 850°C for 1 h. X-ray diffraction analysis revealed that CuO-NiO composite powders were completely converted to Cu-Ni alloy without any reaction phases by hydrogen reduction. The sintered samples showed large and aligned parallel pores to the camphene growth direction, and small pores in the internal wall of large pores. The pore size and porosity decreased with increase in CuO-NiO content from 5 to 10 vol%. The change of pore characteristics was explained by the degree of powder rearrangement in slurry and the accumulation behavior of powders in the interdendritic spaces of solidified camphene.
-
Citations
Citations to this article as recorded by- Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
Eun Byeol Choi, Jong-Hyun Lee
Applied Surface Science.2017; 415: 67. CrossRef - Investigation for Microstructure and Hardness of Welded Zone of Cu-Ni Alloy using W92-Ni-Fe Sintering Tool
Tae-Jin Yoon, Sang-Won Park, Myung-Chang Kang, Joong-Suk Noh, Sung-Wook Chung, Chung-Yun Kang
Journal of Korean Powder Metallurgy Institute.2015; 22(3): 181. CrossRef - Controlling Structural and Electrical Properties of Pt Nanopowder-Dispersed SiO2Film
Jae Ho Lee, In Joo Shin, Sung Woo Lee, Hyeong Cheol Kim, Byung Joon Choi
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 355. CrossRef
- Enhancement in electrical conductivity of pastes containing submicron Ag-coated Cu filler with palmitic acid surface modification
- [Korean]
- Synthesis and Antifungal Property of Porous Al2O3 with Dispersions of Cu Nanoparticles
- Ho-Suk Yoo, Min-Sung Kim, Sung-Tag Oh, Chang-Yong Hyun
- J Korean Powder Metall Inst. 2014;21(1):16-20. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.16
- 806 View
- 1 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
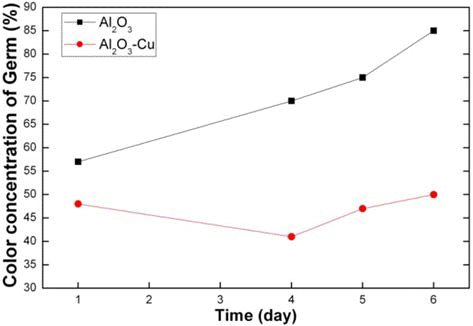
PDF In order to fabricate the porous Al2O3 with dispersion of nano-sized Cu particles, freeze-drying of camphene/ Al2O3 slurry and solution chemistry process using Cu-nitrate are introduced. Camphene slurries with 10 vol% Al2O3 was frozen at -25°C. Pores were generated by sublimation of the camphene during drying in air. The sintered samples at 1400 and 1500°C showed the same size of large pores which were aligned parallel to the sublimable vehicles growth direction. However, the size of fine pores in the internal walls of large pores decreased with increase in sintering temperature. It was shown that Cu particles with the size of 100 nm were homogeneously dispersed on the surfaces of the large pores. Antibacterial test using fungus revealed that the porous Al2O3/1 vol% Cu composite showed antifungal property due to the dispersion of Cu particles. The results are suggested that the porous composites with required pore characteristics and functional property can be fabricated by freeze-drying process and addition of functional nano particles by chemical method.
-
Citations
Citations to this article as recorded by- Collection of Industrial Oil Using Nanoparticles and Porous Powders of Silica
Y.-S. Cho, J.-W. Moon
Archives of Metallurgy and Materials.2017; 62(2): 1371. CrossRef - Fabrication and Mechanical Properties of STS316L Porous Metal for Vacuum Injection Mold
Se Hoon Kim, Sang Min Kim, Sang Ho Noh, Jin Pyeong Kim, Jae Hyuck Shin, Si-Young Sung, Jin Kwang Jin, Taean Kim
Journal of Korean Powder Metallurgy Institute.2015; 22(3): 197. CrossRef
- Collection of Industrial Oil Using Nanoparticles and Porous Powders of Silica
TOP
 KPMI
KPMI


 First
First Prev
Prev


