Search
- Page Path
- HOME > Search
- [Korean]
- Enhancement of the Electrochemical Performance of SiOx Anodes by Al2O3 Coating via Powder Atomic Layer Deposition
- Donggeon Shin, Yoonsoo Han
- J Powder Mater. 2025;32(6):501-508. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00416
- 465 View
- 2 Download
-
 Abstract
Abstract
 PDF
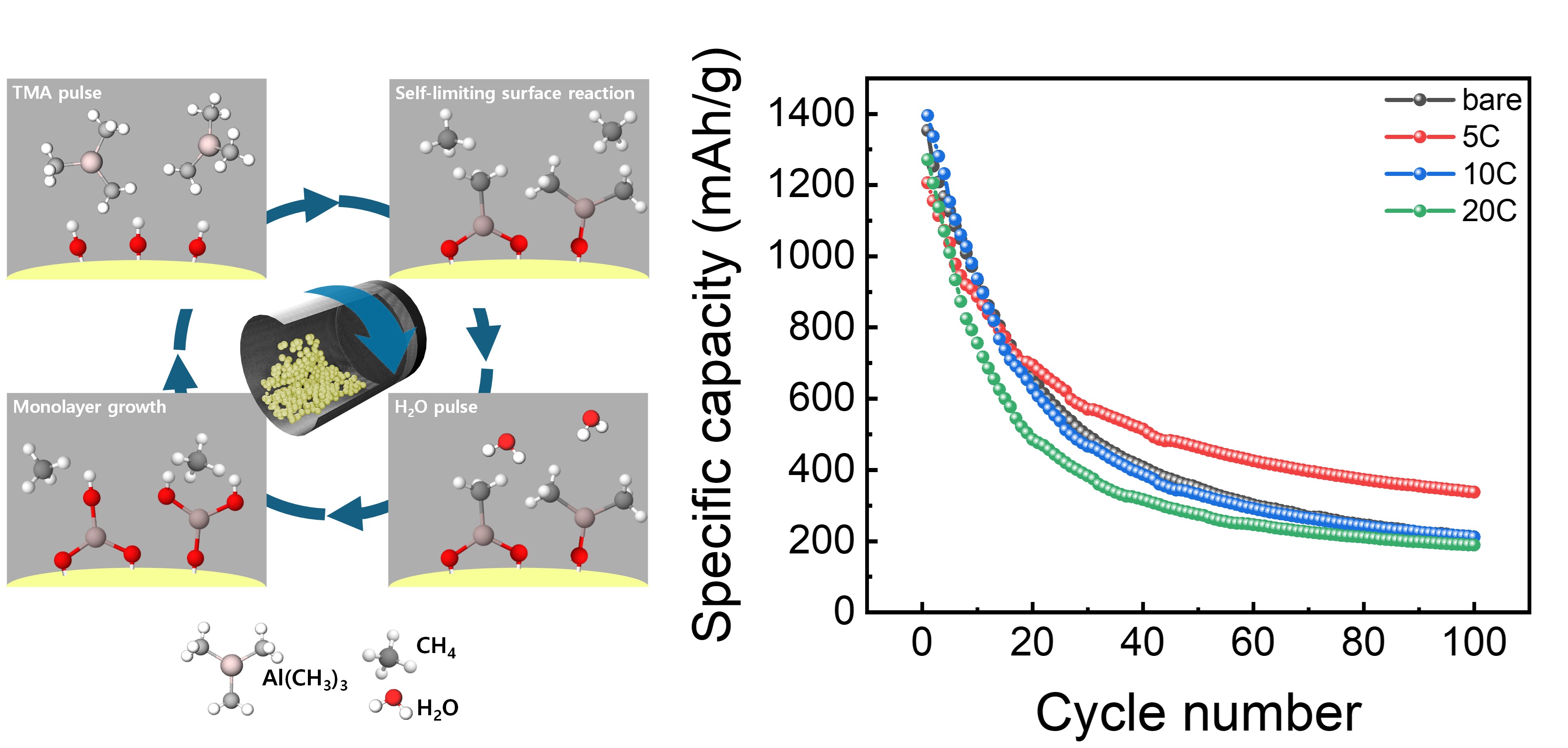
PDF - Silicon based anode materials offer high theoretical capacity but suffer from severe volume expansion and unstable interfacial properties during repeated lithiation and delithiation, resulting in rapid performance degradation. In this study, a thin aluminum oxide coating layer was deposited on Si/SiOx Carbon anode materials using a powder atomic layer deposition (PALD) process to address these limitations. EDS mapping and XRD analyses confirmed the uniform formation of an amorphous aluminum oxide coating with increasing thickness as the deposition cycles increased. Electrochemical evaluation showed that the electrode coated with 5 PALD cycles exhibited approximately 78% higher capacity retention after 100 cycles at 1 A g-1 and a higher initial Coulombic efficiency compared to the bare electrode. The coated electrode also delivered approximately 22% higher capacity at a high current density of 5 A g-1, indicating enhanced rate capability. Cyclic voltammetry analysis revealed increased surface controlled reaction contributions and improved reaction kinetics. These results demonstrate that PALD derived aluminum oxide coatings effectively stabilize the electrode electrolyte interface and enhance the electrochemical performance of silicon based anodes, highlighting their potential for next generation high capacity lithium ion batteries. generation high capacity lithium ion battery anode materials.
- [Korean]
- Fabrication of 3D Aligned h-BN based Polymer Composites with Enhanced Mechanical Properties for Battery Housing
- Kiho Song, Hyunseung Song, Sang In Lee, Changui Ahn
- J Powder Mater. 2024;31(4):329-335. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00220
- 1,217 View
- 31 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
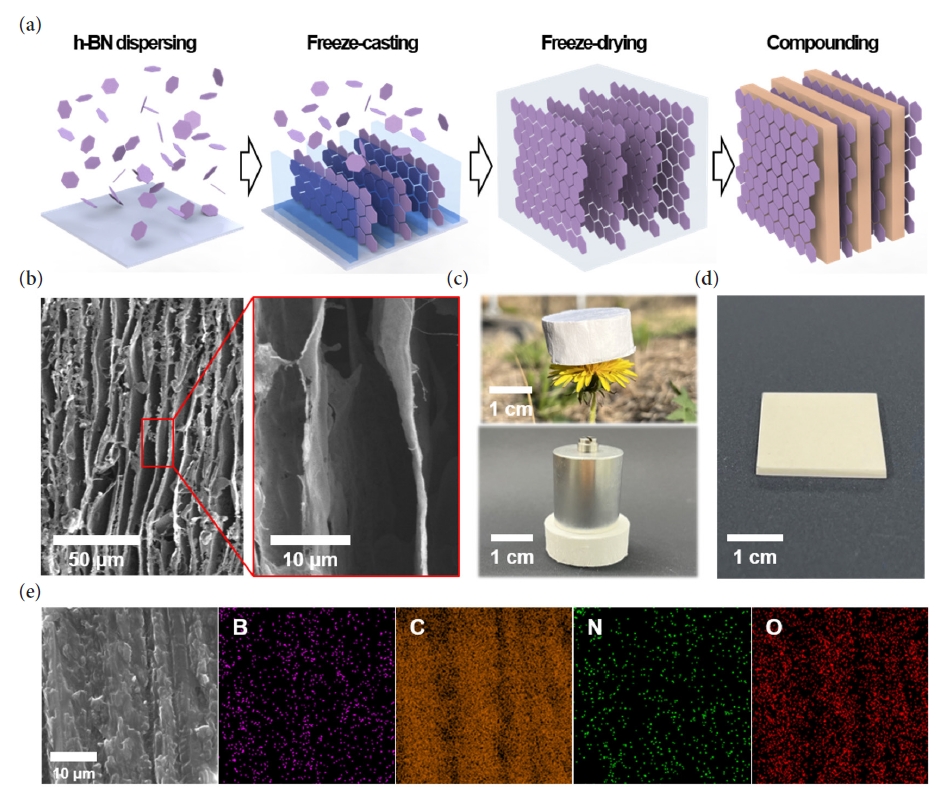
Supplementary Material - As the demand for electric vehicles increases, the stability of batteries has become one of the most significant issues. The battery housing, which protects the battery from external stimuli such as vibration, shock, and heat, is the crucial element in resolving safety problems. Conventional metal battery housings are being converted into polymer composites due to their lightweight and improved corrosion resistance to moisture. The transition to polymer composites requires high mechanical strength, electrical insulation, and thermal stability. In this paper, we proposes a high-strength nanocomposite made by infiltrating epoxy into a 3D aligned h-BN structure. The developed 3D aligned h-BN/epoxy composite not only exhibits a high compressive strength (108 MPa) but also demonstrates excellent electrical insulation and thermal stability, with a stable electrical resistivity at 200 °C and a low thermal expansion coefficient (11.46ⅹppm/℃), respectively.
- [Korean]
- Hydrogen Reduction Behavior of NCM-based Lithium-ion Battery Cathode Materials
- So-Yeong Lee, So-Yeon Lee, Dae-Hyeon Lee, Ho-Sang Sohn
- J Powder Mater. 2024;31(2):163-168. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00017
- 1,412 View
- 38 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
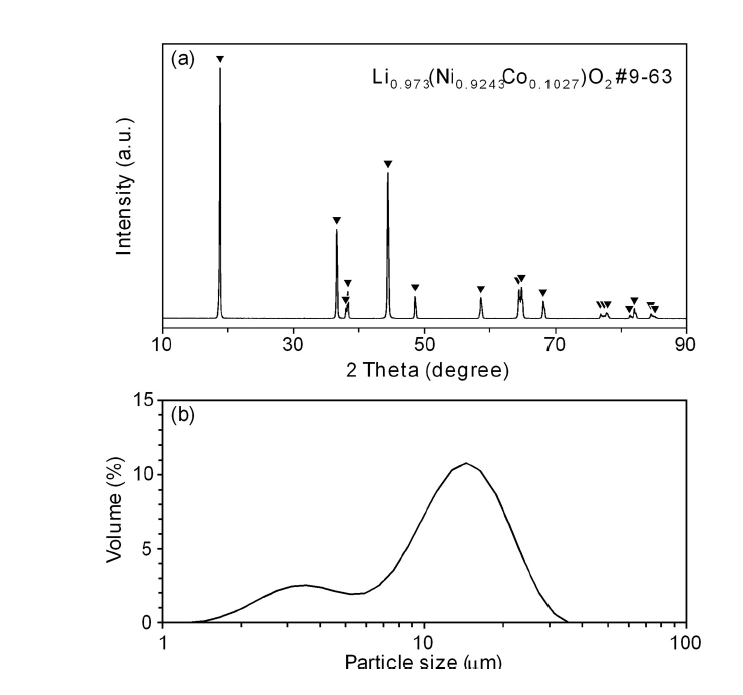
PDF - As the demand for lithium-ion batteries for electric vehicles is increasing, it is important to recover valuable metals from waste lithium-ion batteries. In this study, the effects of gas flow rate and hydrogen partial pressure on hydrogen reduction of NCM-based lithium-ion battery cathode materials were investigated. As the gas flow rate and hydrogen partial pressure increased, the weight loss rate increased significantly from the beginning of the reaction due to the reduction of NiO and CoO by hydrogen. At 700 °C and hydrogen partial pressure above 0.5 atm, Ni and Li2O were produced by hydrogen reduction. From the reduction product and Li recovery rate, the hydrogen reduction of NCM-based cathode materials was significantly affected by hydrogen partial pressure. The Li compounds recovered from the solution after water leaching of the reduction products were LiOH, LiOH·H2O, and Li2CO3, with about 0.02 wt% Al as an impurity.
-
Citations
Citations to this article as recorded by- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef
- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
- [Korean]
- Synthesis of Carbon Coated Nickel Cobalt Sulfide Yolk-shell Microsphere and Their Application as Anode Materials for Sodium Ion Batteries
- Hyo Yeong Seo, Gi Dae Park
- J Powder Mater. 2023;30(5):387-393. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.387
- 688 View
- 14 Download
-
 Abstract
Abstract
 PDF
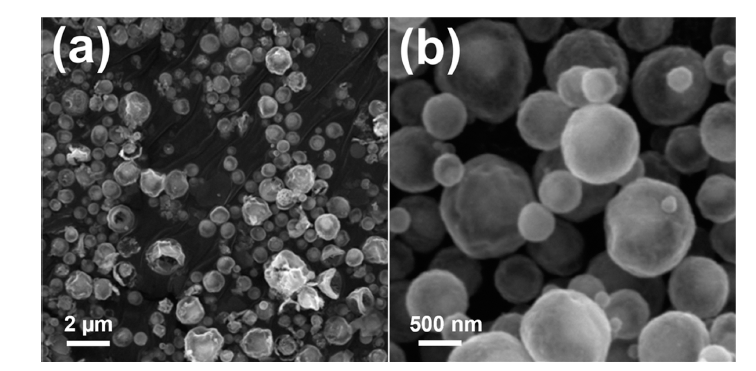
PDF Transition metal chalcogenides are promising cathode materials for next-generation battery systems, particularly sodium-ion batteries. Ni3Co6S8-pitch-derived carbon composite microspheres with a yolk-shell structure (Ni3Co6S8@C-YS) were synthesized through a three-step process: spray pyrolysis, pitch coating, and post-heat treatment process. Ni3Co6S8@C-YS exhibited an impressive reversible capacity of 525.2 mA h g-1 at a current density of 0.5 A g-1 over 50 cycles when employed as an anode material for sodium-ion batteries. However, Ni3Co6S8 yolk shell nanopowder (Ni3Co6S8-YS) without pitch-derived carbon demonstrated a continuous decrease in capacity during charging and discharging. The superior sodium-ion storage properties of Ni3Co6S8@C-YS were attributed to the pitchderived carbon, which effectively adjusted the size and distribution of nanocrystals. The carbon-coated yolk-shell microspheres proposed here hold potential for various metal chalcogenide compounds and can be applied to various fields, including the energy storage field.
- [Korean]
- The Synthesis of Lithium Lanthanum Titanium Oxide for Solid Electrolyte via Ultrasonic Spray Pyrolysis
- Jaeseok Roh, MinHo Yang, Kun-Jae Lee
- J Powder Mater. 2022;29(6):485-491. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.485

- 1,379 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF Lithium lanthanum titanium oxide (LLTO) is a promising ceramic electrolyte because of its high ionic conductivity at room temperature, low electrical conductivity, and outstanding physical properties. Several routes for the synthesis of bulk LLTO are known, in particular, solid-state synthesis and sol-gel method. However, the extremely low ionic conductivity of LLTO at grain boundaries is one of the major problems for practical applications. To diminish the grain boundary effect, the structure of LLTO is tuned to nanoscale morphology with structures of different dimensionalities (0D spheres, and 1D tubes and wires); this strategy has great potential to enhance the ion conduction by intensifying Li diffusion and minimizing the grain boundary resistance. Therefore, in this work, 0D spherical LLTO is synthesized using ultrasonic spray pyrolysis (USP). The USP method primarily yields spherical particles from the droplets generated by ultrasonic waves passed through several heating zones. LLTO is synthesized using USP, and the effects of each precursor and their mechanisms as well as synthesis parameters are analyzed and discussed to optimize the synthesis. The phase structure of the obtained materials is analyzed using X-ray diffraction, and their morphology and particle size are analyzed using field-emission scanning electron microscopy.
- [Korean]
- Electrochemical Properties of Ball-milled Tin-Graphite Composite Anode Materials for Lithium-Ion Battery
- Tae-Hui Lee, Hyeon-A Hong, Kwon-Koo Cho, Yoo-Young Kim
- J Korean Powder Metall Inst. 2021;28(6):462-469. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.462

- 1,069 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF Tin/graphite composites are prepared as anode materials for Li-ion batteries using a dry ball-milling process. The main experimental variables in this work are the ball milling time (0–8 h) and composition ratio (tin:graphite=5:95, 15:85, and 30:70 w/w) of graphite and tin powder. For comparison, a tin/graphite composite is prepared using wet ball milling. The morphology and structure of the different tin/graphite composites are investigated using X-ray diffraction, Raman spectroscopy, energy-dispersive X-ray spectroscopy, and scanning and transmission electron microscopy. The electrochemical properties of the samples are also examined. The optimal dry ball milling time for the uniform mixing of graphite and tin is 6 h in a graphite-30wt.%Sn sample. The electrode prepared from the composite that is dry-ballmilled for 6 h exhibits the best cycle performance (discharge capacity after 50th cycle: 308 mAh/g and capacity retention: 46%). The discharge capacity after the 50th cycle is approximately 112 mAh/g, higher than that when the electrode is composed of only graphite (196 mAh/g after 50th cycle). This result indicates that it is possible to manufacture a tin/graphite composite anode material that can effectively buffer the volume change that occurs during cycling, even using a simple dry ball-milling process.
- [Korean]
- Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
- Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2019;26(1):49-57. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.49
- 1,830 View
- 17 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
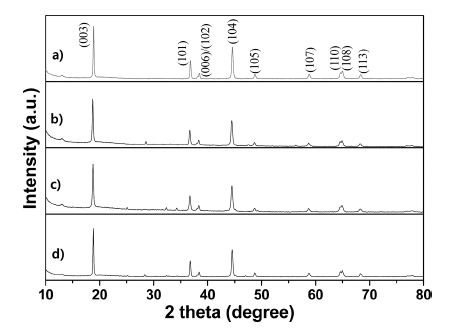
PDF Layered LiNi0.83Co0.11Mn0.06O2 cathode materials single- and dual-doped by the rare-earth elements Ce and Nd are successfully fabricated by using a coprecipitation-assisted solid-phase method. For comparison purposes, nondoping pristine LiNi0.83Co0.11Mn0.06O2 cathode material is also prepared using the same method. The crystal structure, morphology, and electrochemical performances are characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometer (EDS) mapping, and electrochemical techniques. The XRD data demonstrates that all prepared samples maintain a typical α-NaFeO2-layered structure with the
R-3m -
Citations
Citations to this article as recorded by- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
Heewon Choi, Nam-gyu Lim, Seong Jun Lee, Jungsoo Park
Journal of Mechanical Science and Technology.2021; 35(6): 2697. CrossRef - Synthesis of CeVO4-V2O5 nanowires by cation-exchange method for high-performance lithium-ion battery electrode
Xueliu Xu, Shiying Chang, Taofang Zeng, Yidan Luo, Dong Fang, Ming Xie, Jianhong Yi
Journal of Alloys and Compounds.2021; 887: 161237. CrossRef
- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
- [Korean]
- Effect of Auxetic Structure of PVdF on Tin Anode Stability for Na-ion Batteries
- Jinsoo Park
- J Korean Powder Metall Inst. 2018;25(6):507-513. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.507
- 888 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
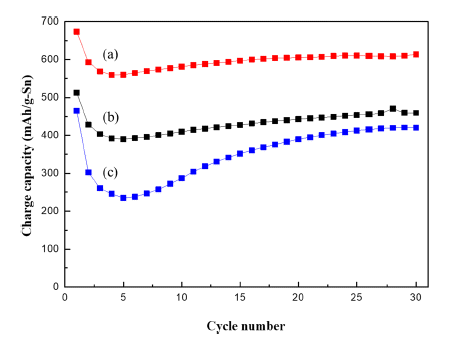
PDF This study investigates the viability of using a Na-ion battery with a tin(Sn) anode to mitigate the vulnerability caused by volume changes during discharge and charge cycling. In general, the volume changes of carbon material do not cause any instability during intercalation into its layer structure. Sn has a high theoretical capacity of 847 mAh g−1. However, it expands dramatically in the discharge process by alloying Na-Sn, placing the electrode under massive internal stress, and particularly straining the binder over the elastic limit. The repeating strain results in loss of active material and its electric contact, as well as capacity decrease. This paper expands the scope of fabrication of Na-ion batteries with Sn by fabricating the binder as an auxetic structure with a unique feature: a negative Poisson ratio (NPR), which increases the resistance to internal stress in the Na-Sn alloying/de-alloying processes. Electrochemical tests and micrograph images of auxetic and common binders are used to compare dimensional and structural differences. Results show that the capacity of an auxetic-structured Sn electrode is much larger than that of a Sn electrode with a common-structured binder. Furthermore, using an auxetic structured Sn electrode, stability in discharge and charge cycling is obtained.
-
Citations
Citations to this article as recorded by- Highly Flexible and Conductive Electrodes through Combining Honeycomb and Butterfly Pattern Bio‐Inspired Structure for ECG Signal Recording
Qi Hou, Min Wang, Chunyang Han, Kuiyang Gao, Ruiyao Liu, Guofeng Yao
Advanced Materials Interfaces.2022;[Epub] CrossRef
- Highly Flexible and Conductive Electrodes through Combining Honeycomb and Butterfly Pattern Bio‐Inspired Structure for ECG Signal Recording
- [English]
- A Separator with Activated Carbon Powder Layer to Enhance the Performance of Lithium-Sulfur Batteries
- Duc-Luong Vu, Jae-Won Lee
- J Korean Powder Metall Inst. 2018;25(6):466-474. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.466
- 971 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
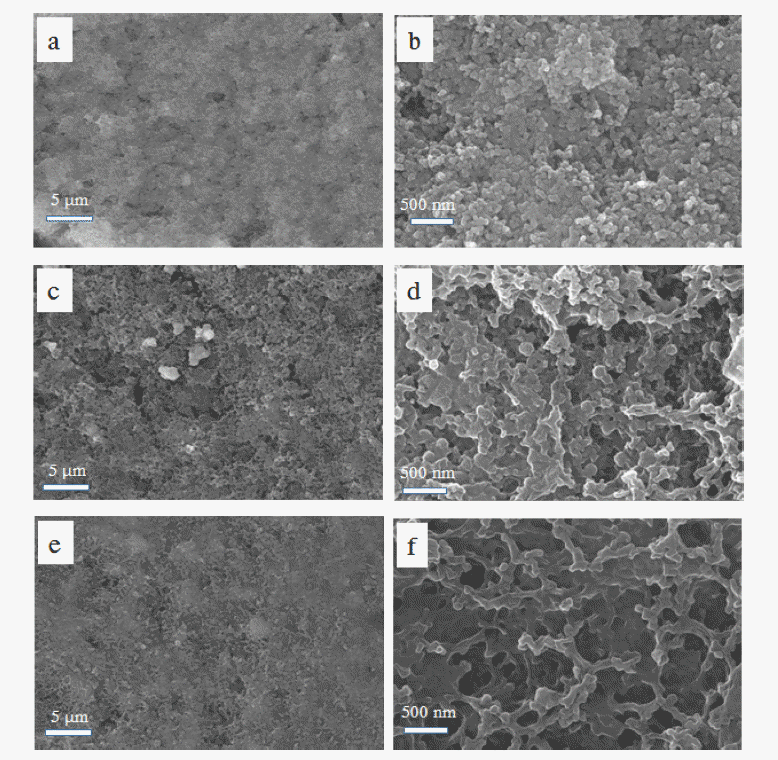
PDF The high theoretical energy density (2600 Wh kg−1) of Lithium-sulfur batteries and the high theoretical capacity of elemental sulfur (1672 mAh g−1) attract significant research attention. However, the poor electrical conductivity of sulfur and the polysulfide shuttle effect are chronic problems resulting in low sulfur utilization and poor cycling stability. In this study, we address these problems by coating a polyethylene separator with a layer of activated carbon powder. A lithium-sulfur cell containing the activated carbon powder-coated separator exhibits an initial specific discharge capacity of 1400 mAh g−1 at 0.1 C, and retains 63% of the initial capacity after 100 cycles at 0.2 C, whereas the equivalent cell with a bare separator exhibits a 1200 mAh g−1 initial specific discharge capacity, and 50% capacity retention under the same conditions. The activated carbon powder-coated separator also enhances the rate capability. These results indicate that the microstructure of the activated carbon powder layer provides space for the sulfur redox reaction and facilitates fast electron transport. Concurrently, the activated carbon powder layer traps and reutilizes any polysulfides dissolved in the electrolyte. The approach presented here provides insights for overcoming the problems associated with lithium-sulfur batteries and promoting their practical use.
-
Citations
Citations to this article as recorded by- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
Yueting Zhu, Jingjing Wang, Yanshu Wang, Ying Zhu, Yixuan Li, Shicheng Zhao
Ionics.2022; 28(4): 1693. CrossRef - High thermal stability multilayered electrolyte complexes via layer-by-layer for long-life lithium-sulfur battery
Jing Wang, Yufan Li, Xianmei Deng, Lei Yan, Zhiqiang Shi
Ionics.2020; 26(11): 5481. CrossRef
- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
- [Korean]
- Preparation of Sintering Aid for Li7La3Zr2O12 Solid Electrolyte by Heat-treatment of Polymeric Precursors Containing Li and B
- Ran-Hee Shin, Sung-Soo Ryu
- J Korean Powder Metall Inst. 2018;25(2):151-157. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.151

- 832 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, the compound Li3BO3 (LBO) is intended to be prepared by a polymeric complex method as a sintering aid for the densification of Li7La3Zr2O12 (LLZ) solid electrolyte. A polymeric precursor containing Li and B is heat-treated in an air atmosphere at a temperature range between 600°C and 800°C. Instead of LBO, the compound Li2+xC1-xBxO3 (LCBO) is unexpectedly synthesized after a heat-treatment of 700°C. The effect of LCBO addition on sintering behavior and ion conductivity of LLZ is studied. It is found that the LCBO compound could lead to significant improvements in the densification and ionic conductivity of LLZ compared to pure LLZ. After sintering at 1100°C, the density of the LLZ-12wt%LBO composite is 3.72 g/cm3, with a high Li-ion conductivity of 1.18 × 10−4 Scm-1 at 28°C, while the pure LLZ specimen had a densify of 2.98 g/cm3 and Li-ion conductivity of 5.98 × 10−6 Scm-1.
-
Citations
Citations to this article as recorded by- Characterization of Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte with an Added Sintering Aid
Hyun-Joon Lee, Liyu-Liu, Won-Jong Jeong, Seoung-Ki Lee, Bong-Ki Ryu
Electronic Materials Letters.2023; 19(1): 55. CrossRef
- Characterization of Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte with an Added Sintering Aid
- [English]
- Fabrication and Characterization of Thermal Battery using Porous MgO Separator Infiltrated with Li based Molten Salts
- Kyungho Kim, Sungmin Lee, Chae-Nam Im, Seung-Ho Kang, Hae-Won Cheong, Yoonsoo Han
- J Korean Powder Metall Inst. 2017;24(5):364-369. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.364
- 732 View
- 9 Download
-
 Abstract
Abstract
 PDF
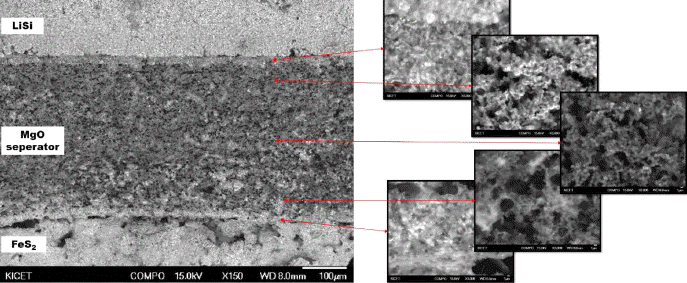
PDF Ceramic powder, such as MgO, is added as a binder to prepare the green compacts of molten salts of an electrolyte for a thermal battery. Despite the addition of a binder, when the thickness of the electrolyte decreases to improve the battery performance, the problem with the unintentional short circuit between the anode and cathode still remains. To improve the current powder molding method, a new type of electrolyte separator with porous MgO preforms is prepared and characteristics of the thermal battery are evaluated. A Spherical PMMA polymer powder is added as a pore-forming agent in the MgO powder, and an organic binder is used to prepare slurry appropriate for tape casting. A porous MgO preform with 300 μm thickness is prepared through a binder burnout and sintering process. The particle size of the starting MgO powder has an effect, not on the porosity of the porous MgO preform, but on the battery characteristics. The porosity of the porous MgO preforms is controlled from 60 to 75% using a pore-forming agent. The batteries prepared using various porosities of preforms show a performance equal to or higher than that of the pellet-shaped battery prepared by the conventional powder molding method.
- [Korean]
- Fabrication of Fe3O4/Fe/Graphene nanocomposite powder by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ji-Seub Choi, Hoi-Jin Lee, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2017;24(4):308-314. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.308
- 936 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
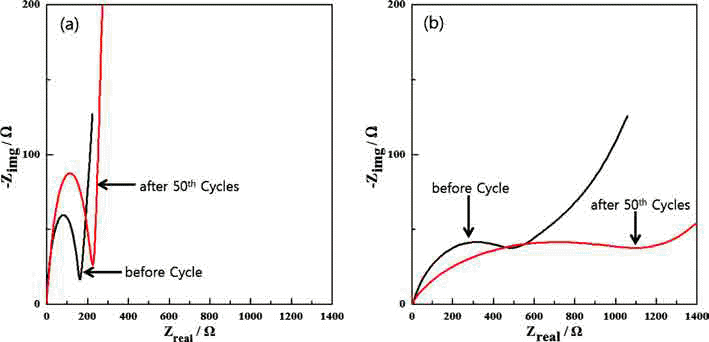
PDF Fe3O4/Fe/graphene nanocomposite powder is synthesized by electrical wire explosion of Fe wire and dispersed graphene in deionized water at room temperature. The structural and electrochemical characteristics of the powder are characterized by the field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, field-emission transmission electron microscopy, cyclic voltammetry, and galvanometric discharge-charge method. For comparison, Fe3O4/Fe nanocomposites are fabricated under the same conditions. The Fe3O4/Fe nanocomposite particles, around 15-30 nm in size, are highly encapsulated in a graphene matrix. The Fe3O4/Fe/graphene nanocomposite powder exhibits a high initial charge specific capacity of 878 mA/g and a high capacity retention of 91% (798 mA/g) after 50 cycles. The good electrochemical performance of the Fe3O4/Fe/graphene nanocomposite powder is clearly established by comparison of the results with those obtained for Fe3O4/Fe nanocomposite powder and is attributed to alleviation of volume change, good distribution of electrode active materials, and improved electrical conductivity upon the addition of graphene.
-
Citations
Citations to this article as recorded by- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
Kyunbae Lee, Joonsik Lee, Byung Mun Jung, Byeongjin Park, Taehoon Kim, Sang Bok Lee
Applied Surface Science.2019; 478: 733. CrossRef
- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
- [English]
- Expansion of Multi-wall Carbon Nanotubes and its Lithium Storage Property
- Jung-Ho Ahn, Jeong-Seok Ahn
- J Korean Powder Metall Inst. 2017;24(4):275-278. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.275
- 698 View
- 2 Download
-
 Abstract
Abstract
 PDF
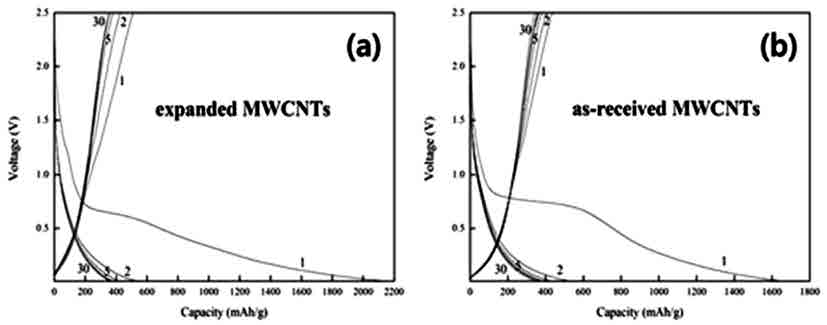
PDF In the present work, we apply a technique that has been used for the expansion of graphite to multiwall carbon nanotubes (MWCNT). The nanotubes are rapidly heated for a short duration, followed by immersion in acid solution, so that they undergo expansion. The diameter of the expanded CNTs is 5-10 times larger than that of the asreceived nanotubes. This results in considerable swelling of the CNTs and opening of the tube tips, which may facilitate the accessibility of lithium ions into the inner holes and the interstices between the nanotube walls. The Li-ion storage capacity of the expanded nanotubes is measured by using the material as an anode in Li-ion cells. The result show that the discharge capacity of the expanded nanotubes in the first cycle is as high as 2,160 mAh/g, which is about 28% higher than that of the un-treated MWCNT anode. However, the charge/discharge capacity quickly drops in subsequent cycles and finally reaches equilibrium values of ~370 mAh/g. This is possibly due to the destruction of the lattice structures by repeated intercalation of Li ions.
- [Korean]
- Effects of Porous Microstructure on the Electrochemical Properties of Si-Ge-Al Base Anode Materials for Li-ion Rechargeable Batteries
- Chung Rae Cho, Myeong Geun Kim, Keun Yong Sohn, Won-Wook Park
- J Korean Powder Metall Inst. 2017;24(1):24-28. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.24
- 573 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Silicon alloys are considered promising anode active materials to replace Li-ion batteries by graphite powder, because they have a relatively high capacity of up to 4200 mAh/g, and are environmentally friendly and inexpensive ECO-materials. However, its poor charge/discharge properties, induced by cracking during cycles, constitute their most serious problem as anode electrode. In order to solve these problems, Si-Ge-Al alloys with porous structure are designed as anode alloy powders, to improve cycling stability. The alloys are melt-spun to obtain the rapidly solidified ribbons, and then ball-milled to make fine powders. The powders are etched using 1 M HCl solution, which gives the powders a porous structure by removing the element Al. Subsequently, in this study, the microstructures and the characteristics of the etched powders are evaluated for application as anode materials. As a result, the etched porous powder shows better electrochemical properties than as-milled Si-Ge-Al powder.
- [English]
- Representative Volume Element Analysis of Fluid-Structure Interaction Effect on Graphite Powder Based Active Material for Lithium-Ion Batteries
- Jin Chul Yun, Seong Jin Park
- J Korean Powder Metall Inst. 2017;24(1):17-23. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.17

- 605 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF In this study, a finite element analysis approach is proposed to predict the fluid-structure interaction behavior of active materials for lithium-ion batteries (LIBs), which are mainly composed of graphite powder. The porous matrix of graphite powder saturated with fluid electrolyte is considered a representative volume element (RVE) model. Three different RVE models are proposed to consider the uncertainty of the powder shape and the porosity. Pwave modulus from RVE solutions are analyzed based on the microstructure and the interaction between the fluid and the graphite powder matrix. From the results, it is found that the large surface area of the active material results in low mechanical properties of LIB, which leads to poor structural durability when subjected to dynamic loads. The results obtained in this study provide useful information for predicting the mechanical safety of a battery pack.
- [Korean]
- Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
- Woonghee Choi, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(4):287-296. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.287
- 1,634 View
- 31 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Ni1/3Co1/3Mn1/3(OH)2 powders have been synthesized in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH using NH4OH as a chelating agent. The co-precipitation temperature is varied in the range of 30-80°C. Calcination of the prepared precursors with Li2CO3 for 8 h at 1000°C in air results in Li Ni1/3Co1/3Mn1/3O2 powders. Two kinds of obtained powders have been characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analyzer, and tap density measurements. The co-precipitation temperature does not differentiate the XRD patterns of precursors as well as their final powders. Precursor powders are spherical and dense, consisting of numerous acicular or flaky primary particles. The precursors obtained at 70 and 80°C possess bigger primary particles having more irregular shapes than those at lower temperatures. This is related to the lower tap density measured for the former. The final powders show a similar tendency in terms of primary particle shape and tap density. Electrochemical characterization shows that the initial charge/discharge capacities and cycle life of final powders from the precursors obtained at 70 and 80°C are inferior to those at 50°C. It is concluded that the optimum co-precipitation temperature is around 50°C.
-
Citations
Citations to this article as recorded by- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
Seon Hwa Lee, Ki Young Kwon, Byeong Kil Choi, Hyun Deog Yoo
Journal of Electroanalytical Chemistry.2022; 924: 116828. CrossRef
- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
- [Korean]
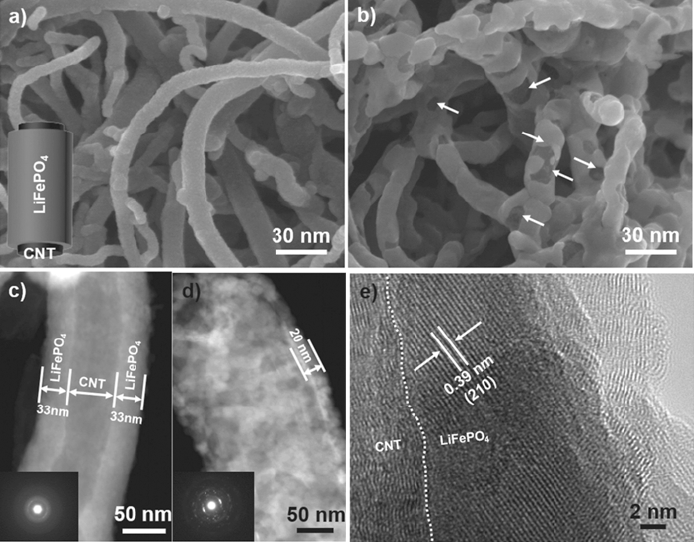
- Recent Progress on the Application of Atomic Layer Deposition for Lithium Ion Batteries
- Dong Ha Kim, Byung Joon Choi
- J Korean Powder Metall Inst. 2016;23(2):170-176. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.170
- 2,456 View
- 29 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Lithium-ion batteries (LIBs) are rapidly improving in capacity and life cycle characteristics to meet the requirements of a wide range of applications, such as portable electronics, electric vehicles, and micro- or nanoelectromechanical systems. Recently, atomic layer deposition (ALD), one of the vapor deposition methods, has been explored to expand the capability of LIBs by producing near-atomically flat and uniform coatings on the shell of nanostructured electrodes and membranes for conventional LIBs. In this paper, we introduce various ALD coatings on the anode, cathode, and separator materials to protect them and improve their electrochemical and thermomechanical stability. In addition, we discuss the effects of ALD coatings on the three-dimensional structuring and conduction layer through activation of electrochemical reactions and facilitation of fluent charge collection.
-
Citations
Citations to this article as recorded by- Atomic Layer Deposition for Powder Coating
Seok Choi, Jeong Hwan Han, Byung Joon Choi
Journal of Korean Powder Metallurgy Institute.2019; 26(3): 243. CrossRef - Formation of Uniform SnO2 Coating Layer on Carbon Nanofiber by Pretreatment in Atomic Layer Deposition
Dong Ha Kim, Doh-Hyung Riu, Byung Joon Choi
journal of Korean Powder Metallurgy Institute.2018; 25(1): 43. CrossRef
- Atomic Layer Deposition for Powder Coating
- [Korean]
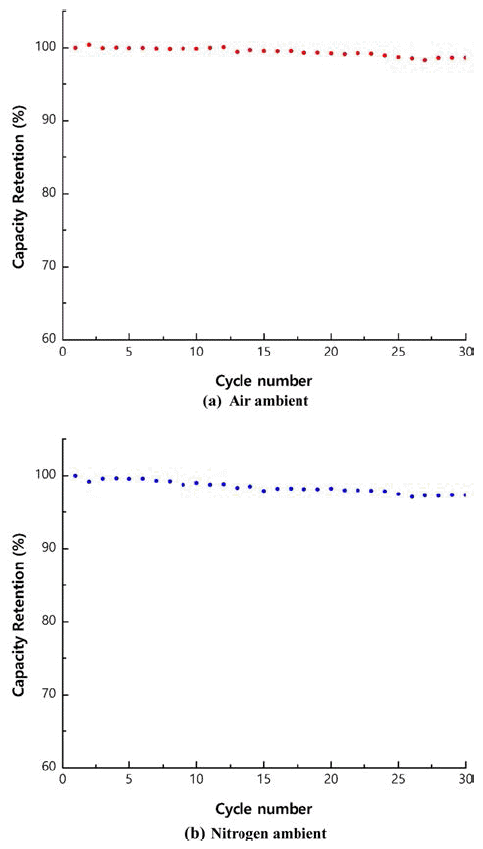
- Characteristics of Ni1/3Co1/3Mn1/3(OH)2 Powders Prepared by Co-Precipitation in Air and Nitrogen Atmospheres
- Woonghee Choi, Se-Ryen Park, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(2):136-142. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.136
- 2,458 View
- 45 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF As precursors of cathode materials for lithium ion batteries, Ni1/3Co1/3Mn1/3(OH)2 powders are prepared in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH in the presence of NH4OH in air or nitrogen ambient. Calcination of the precursors with Li2CO3 for 8 h at 1,000°C in air produces dense spherical cathode materials. The precursors and final powders are characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analysis, tap density measurement, and thermal gravimetric analysis. The precursor powders obtained in air or nitrogen ambient show XRD patterns identified as Ni1/3Co1/3Mn1/3(OH)2. Regardless of the atmosphere, the final powders exhibit the XRD patterns of LiNi1/3Co1/3Mn1/3O2 (NCM). The precursor powders obtained in air have larger particle size and lower tap density than those obtained in nitrogen ambient. NCM powders show similar tendencies in terms of particle size and tap density. Electrochemical characterization is performed after fabricating a coin cell using NCM as the cathode and Li metal as the anode. The NCM powders from the precursors obtained in air and those from the precursors obtained in nitrogen have similar initial charge/discharge capacities and cycle life. In conclusion, the powders co-precipitated in air can be utilized as precursor materials, replacing those synthesized in the presence of nitrogen injection, which is the usual industrial practice.
-
Citations
Citations to this article as recorded by- Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
Minjeong Kim, Soonhyun Hong, Heongkwon Jeon, Jahun Koo, Heesang Lee, Gyuseok Choi, Chunjoong Kim
Korean Journal of Materials Research.2022; 32(4): 216. CrossRef - Spherical agglomeration of nickel-manganese-cobalt hydroxide in turbulent Batchelor vortex flow
Xiaotong Sun, Jinsoo Kim, Woo-Sik Kim
Chemical Engineering Journal.2021; 421: 129924. CrossRef - Design strategies for development of nickel-rich ternary lithium-ion battery
Kyu Hwan Choi, Xuyan Liu, Xiaohong Ding, Qiang Li
Ionics.2020; 26(3): 1063. CrossRef - Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 49. CrossRef - Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
Woonghee Choi, Chan Hyoung Kang
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 287. CrossRef
- Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
- [Korean]
- Shape Control of Anodic Aluminum Oxide and Effect as Support of Silicon Powder Electrode
- Ju-Seok Song, Jong-Keun Ha, Yoo-Young Kim, Dong-Kyu Park, In-Shup Ahn, Jou-Hyeon Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2015;22(4):240-246. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.240

- 647 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Anodic aluminum oxide (AAO) has been widely used for the development and fabrication of nano-powder with various morphologies such as particle, wire, rod, and tube. So far, many researchers have reported about shape control and fabrication of AAO films. However, they have reported on the shape control with different diameter and length of anodic aluminum oxide mainly. We present a combined mild-hard (or hard-mild) anodization to prepare shape-controlled AAO films. Two main parameters which are combination mild-hard (or hard-mild) anodization and run-time of voltage control are applied in this work. The voltages of mild and hard anodization are respectively 40 and 80 V. Anodization was conducted on the aluminum sheet in 0.3 mole oxalic acid at 4°C. AAO films with morphologies of varying interpore distance, branch-shaped pore, diameter-modulated pore and long funnel-shaped pore were fabricated. Those shapes will be able to apply to fabricate novel nano-materials with potential application which is especially a support to prevent volume expansion of inserted active materials, such as metal silicon or tin powder, in lithium ion battery. The silicon powder electrode using an AAO as a support shows outstanding cycle performance as 1003 mAh/g up to 200 cycles.
-
Citations
Citations to this article as recorded by- Nano silicon encapsulated in modified copper as an anode for high performance lithium ion battery
Jong-Keun Ha, Anupriya K. Haridas, Gyu-Bong Cho, Hyo-Jun Ahn, Jou-Hyeon Ahn, Kwon-Koo Cho
Applied Surface Science.2019; 481: 307. CrossRef
- Nano silicon encapsulated in modified copper as an anode for high performance lithium ion battery
- [English]
- Preparation and Characterization of Porous Silicon and Carbon Composite as an Anode Material for Lithium Rechargeable Batteries
- Junsoo Park, Jae-won Lee
- J Korean Powder Metall Inst. 2015;22(1):15-20. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.15
- 678 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The composite of porous silicon (Si) and amorphous carbon (C) is prepared by pyrolysis of a nano-porous Si + pitch mixture. The nano-porous Si is prepared by mechanical milling of magnesium powder with silicon monoxide (SiO) followed by removal of MgO with hydrochloric acid (etching process). The Brunauer-Emmett-Teller (BET) surface area of porous Si (64.52 m2g−1) is much higher than that before etching Si/MgO (4.28 m2g−1) which indicates pores are formed in Si after the etching process. Cycling stability is examined for the nano-porous Si + C composite and the result is compared with the composite of nonporous Si + C. The capacity retention of the former composite is 59.6% after 50 charge/discharge cycles while the latter shows only 28.0%. The pores of Si formed after the etching process is believed to accommodate large volumetric change of Si during charging and discharging process.
- [Korean]
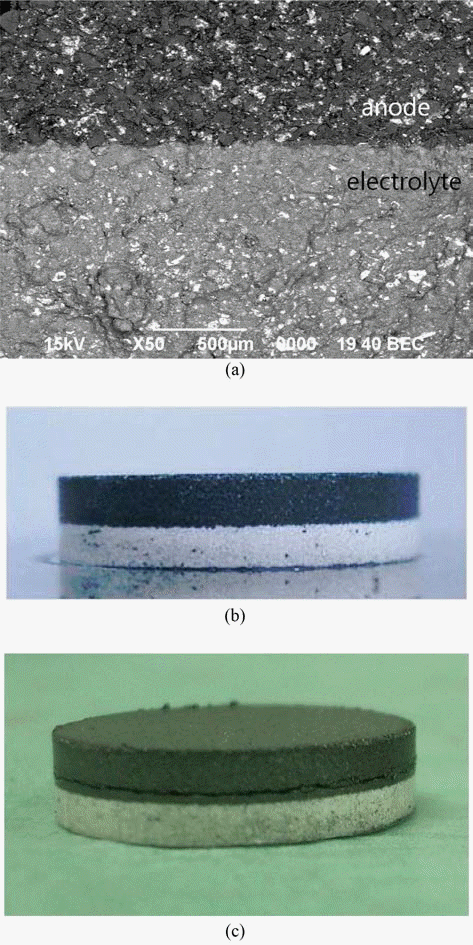
- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
- Sung-Soo Ryu, Hui-Sik Kim, Seongwon Kim, Hyung-Tae Kim, Hae-Won Cheong, Sung-Min Lee
- J Korean Powder Metall Inst. 2014;21(5):331-337. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.331
- 660 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF The effects of particle size of Li-Si alloy and LiCl-KCl addition as a binder phase for raw material of anode were investigated on the formability of the thermal battery anode. The formability was evaluated with respect to filling density, tap density, compaction density, spring-back and compressive strength. With increasing particle size of Li-Si alloy powder, densities increased while spring-back and compressive strength decreased. Since the small spring-back is beneficial to avoiding breakage of pressed compacts, larger particles might be more suitable for anode forming. The increasing amount of LiCl-KCl binder phase contributed to reducing spring-back, improving the formability of anode powder too. The control of particle size also seems to be helpful to get double pressed pellets, which consisted of two layer of anode and electrolyte.
- [Korean]
- Fabrication of LiNiO2 using NiSO4 Recovered from NCM (Li[Ni,Co,Mn]O2) Secondary Battery Scraps and Its Electrochemical Properties
- Yong-Gyu Kwag, Mi-So Kim, Yoo-Young Kim, Im-Sic Choi, Dong-Kyu Park, In-Sup Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2014;21(4):286-293. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.286

- 713 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The electrochemical properties of cells assembled with the LiNiO2 (LNO) recycled from cathode materials of waste lithium secondary batteries (Li[Ni,Co,Mn]O2), were evaluated in this study. The leaching, neutralization and solvent extraction process were applied to produce high-purity NiSO4 solution from waste lithium secondary batteries. High-purity NiO powder was then fabricated by the heat-treatment and mixing of the NiSO4 solution and H2C2O4. Finally, LiNiO2 as a cathode material for lithium ion secondary batteries was synthesized by heat treatment and mixing of the NiO and Li2CO3 powders. We assembled the cells using the LiNiO2 powders and evaluated the electrochemical properties. Subsequently, we evaluated the recycling possibility of the cathode materials for waste lithium secondary battery using the processes applied in this work.
- [Korean]
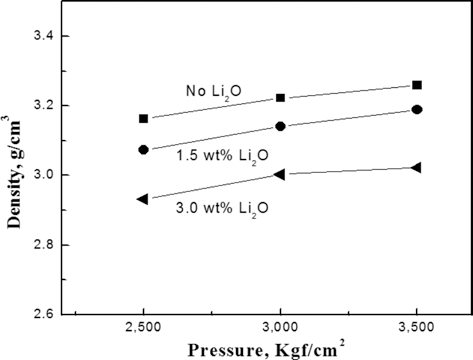
- Effects of Li2O Addition and Heat-Treatment on Formability of FeS2 Powder for Cathode of Thermal Battery
- Sung-Soo Ryu, Won-Jin Lee, Seongwon Kim, Hae-Won Cheong, Sung-Baek Cho, Seung-Ho Kang, Sung-Min Lee
- J Korean Powder Metall Inst. 2014;21(3):185-190. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.185
- 620 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF FeS2 has been widely used for cathode materials in thermal battery because of its high stability and current capability at high operation temperature. Salts such as a LiCl-KCl were added as a binder for improving electrical performance and formability of FeS2 cathode powder. In this study, the effects of the addition of Li2O in LiCl-KCl binder on the formability of FeS2 powder compact were investigated. With the increasing amount of Li2O addition to LiCl-KCl binder salts, the strength of the pressed compacts increased considerably when the powder mixture were pre-heat-treated above 350°C. The heat-treatment resulted in promoting the coating coverage of FeS2 particles by the salts as Li2O was added. The observed coating as Li2O addition might be attributed to the enhanced wettability of the salt rather than its reduced melting temperature. The high strength of compacts by the Li2O addition and pre-heat-treatment could improve the formability of FeS2 raw materials.
-
Citations
Citations to this article as recorded by- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
Sung-Soo Ryu, Hui-Sik Kim, Seongwon Kim, Hyung-Tae Kim, Hae-Won Cheong, Sung-Min Lee
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 331. CrossRef
- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
- Jae-won Lee, Dae Weon Kim, Seong Tae Jang
- J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
- 550 View
- 3 Download
-
 Abstract
Abstract
 PDF
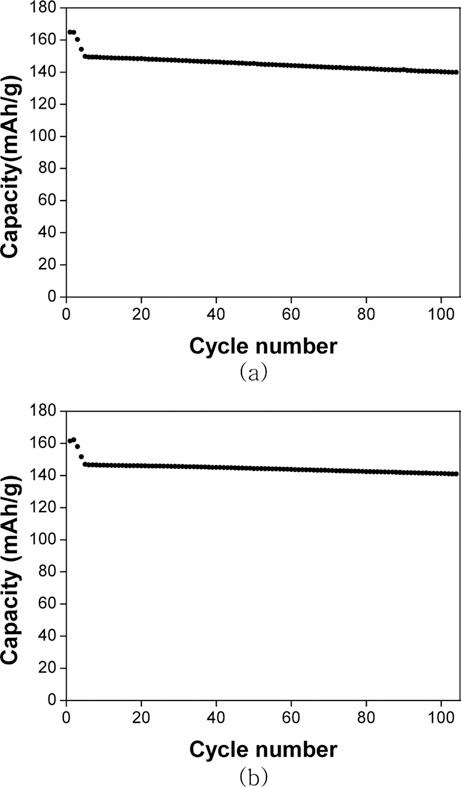
PDF Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
TOP
 KPMI
KPMI


 First
First Prev
Prev


