Search
- Page Path
- HOME > Search
- [English]
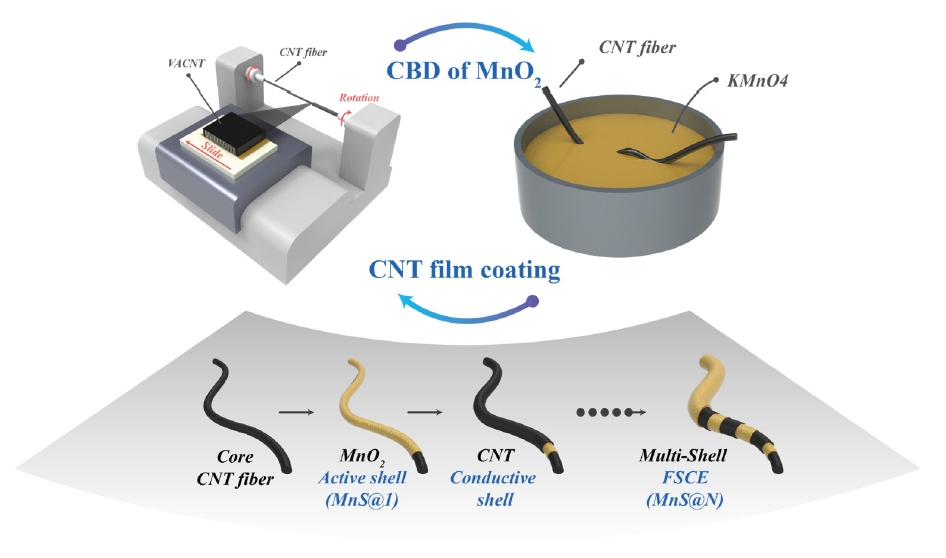
- The Effect of a CNT/MnO2 Nanoparticle Composite–Based Multi-Shell Typed Electrode for a Fiber Supercapacitor (FSC)
- Yeonggwon Kim, Hyung Woo Lee
- J Powder Mater. 2025;32(1):30-36. Published online February 28, 2025
- DOI: https://doi.org/10.4150/jpm.2024.00416
- 1,123 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Fiber supercapacitors have attracted significant interest as potential textile energy storage devices due to their remarkable flexibility and rapid charge/discharge capabilities. This study describes the fabrication of a composite fiber supercapacitor (FSC) electrode through a multi-shell architecture, featuring layers of carbon nanotube (CNT) conductive shells and MnO₂ nanoparticle active shells. The number of layers was adjusted to assess their impact on FSC energy storage performance. Increasing the number of shells reduced electrode resistance and enhanced pseudocapacitive characteristics. Compared to the MnS@1 electrode, the MnS@5 electrode exhibited a high areal capacitance of 301.2 mF/cm², a 411% increase, but showed a higher charge transfer resistance (RCT) of 701.6 Ω. This is attributed to reduced ion diffusion and charge transfer ability resulting from the thicker multi-shell configuration. These results indicate that fine-tuning the quantity of shells is crucial for achieving an optimal balance between energy storage efficiency and stability.
- [English]
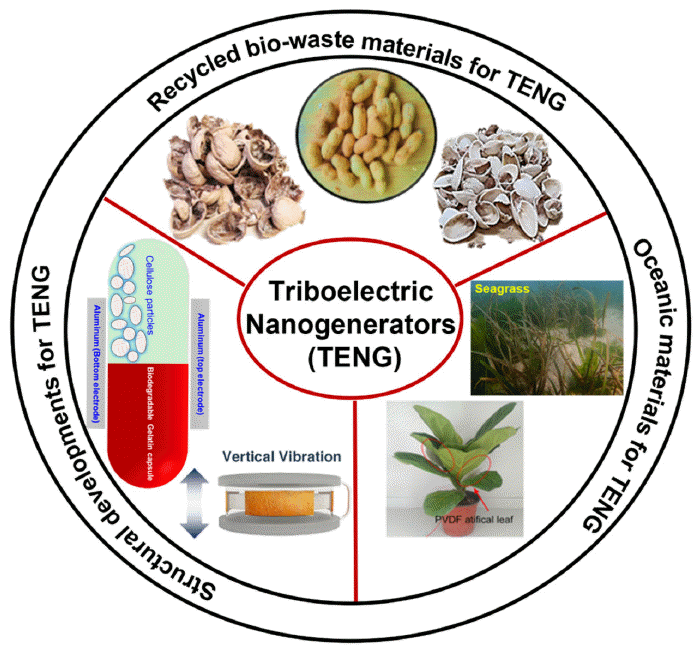
- Eco-Friendly Powder and Particles-Based Triboelectric Energy Harvesters
- Rayyan Ali Shaukat, Jihun Choi, Chang Kyu Jeong
- J Powder Mater. 2023;30(6):528-535. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.528
- 2,026 View
- 38 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF Since their initial development in 2012, triboelectric nanogenerators (TENGs) have gained popularity worldwide as a desired option for harnessing energy. The urgent demand for TENGs is attributed to their novel structural design, low cost, and use of large-scale materials. The output performance of a TENG depends on the surface charge density of the friction layers. Several recycled and biowaste materials have been explored as friction layers to enhance the output performance of TENGs. Natural and oceanic biomaterials have also been investigated as alternatives for improving the performance of TENG devices. Moreover, structural innovations have been made in TENGs to develop highly efficient devices. This review summarizes the recent developments in recycling and biowaste materials for TENG devices. The potential of natural and oceanic biowaste materials is also discussed. Finally, future outlooks for the structural developments in TENG devices are presented.
-
Citations
Citations to this article as recorded by- Triboelectric Nanogenerators for Future Space Missions
Rayyan Ali Shaukat, Muhammad Muqeet Rehman, Maryam Khan, Rui Chang, Carlo Saverio Iorio, Yarjan Abdul Samad, Yijun Shi
Nano-Micro Letters.2026;[Epub] CrossRef - Fabrication and Characterization of a Flexible Polyurethane-Based Triboelectric Nanogenerator for a Harvesting Energy System
Saba Ejaz, Imran Shah, Shahid Aziz, Gul Hassan, Ahmed Shuja, Muhammad Asif Khan, Dong-Won Jung
Micromachines.2025; 16(2): 230. CrossRef - Optimized Process and Mechanical and Electrical Analysis of Polyimide/Pb(Zr,Ti)O3-Based Flexible Piezoelectric Composites
Junki Lee, Sang-il Yoon, Hyunseung Kim, Chang Kyu Jeong
Journal of Powder Materials.2025; 32(1): 16. CrossRef - Sustainable Triboelectric Nanogenerator from Abalone Shell Powder for Self-Powered Humidity Sensing
Yunsook Yang, Farhan Akhtar, Shahzad Iqbal, Muhammad Muqeet Rehman, Woo Young Kim
Sensors.2025; 25(24): 7584. CrossRef - Research Trends in Magneto-Mechano-Electric (MME) Energy Harvesting Devices
So Ie Jeong, Geon-Tae Hwang
Journal of Powder Materials.2025; 32(6): 529. CrossRef
- Triboelectric Nanogenerators for Future Space Missions
- [Korean]
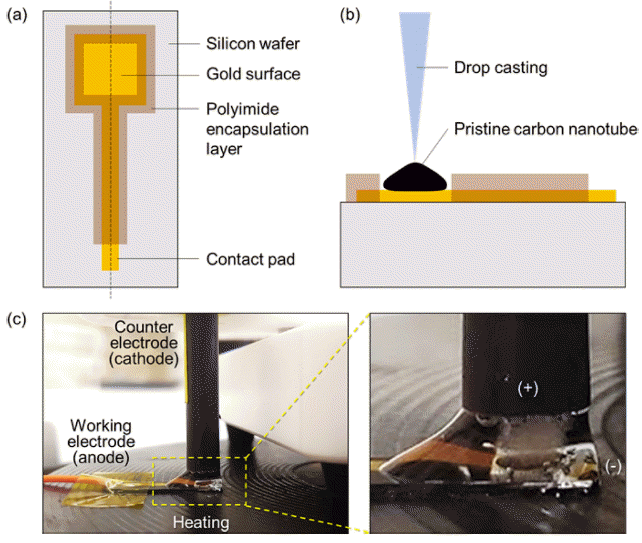
- Capacitance Enhancement and Evaluation of Gold-Deposited Carbon Nanotube Film Ion-Selective Electrode
- Do Youn Kim, Hanbyeol Son, Hyo-Ryoung Lim
- J Powder Mater. 2023;30(4):310-317. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.310
- 952 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF Small-film-type ion sensors are garnering considerable interest in the fields of wearable healthcare and home-based monitoring systems. The performance of these sensors primarily relies on electrode capacitance, often employing nanocomposite materials composed of nano- and sub-micrometer particles. Traditional techniques for enhancing capacitance involve the creation of nanoparticles on film electrodes, which require cost-intensive and complex chemical synthesis processes, followed by additional coating optimization. In this study, we introduce a simple one-step electrochemical method for fabricating gold nanoparticles on a carbon nanotube (Au NP–CNT) electrode surface through cyclic voltammetry deposition. Furthermore, we assess the improvement in capacitance by distinguishing between the electrical double-layer capacitance and diffusion-controlled capacitance, thereby clarifying the principles underpinning the material design. The Au NP–CNT electrode maintains its stability and sensitivity for up to 50 d, signifying its potential for advanced ion sensing. Additionally, integration with a mobile wireless data system highlights the versatility of the sensor for health applications.
- [Korean]
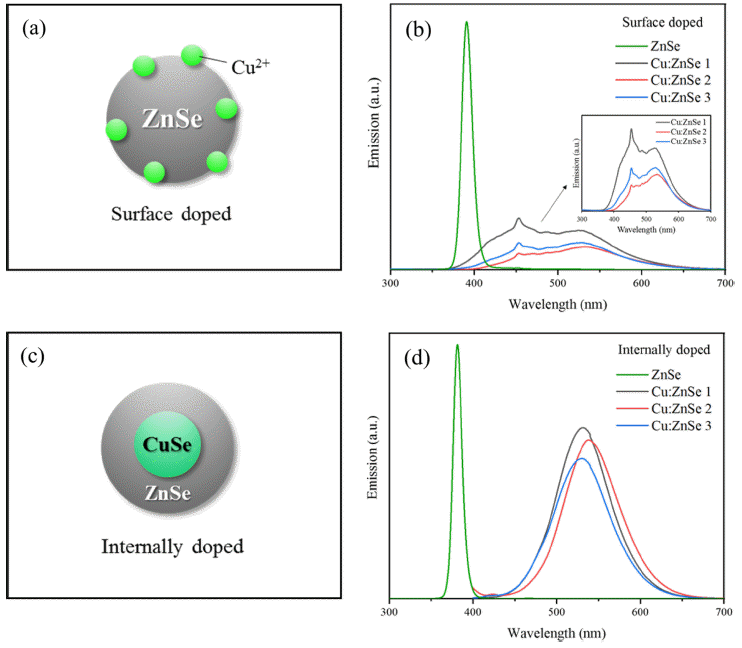
- Aqueous Synthesis and Luminescent Characteristics of Cu:ZnSe Quantum Dots by Internal Doping Method
- Geum Ji Back, Hyun Seon Hong
- J Powder Mater. 2022;29(5):370-375. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.370
- 851 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Cu-doped ZnSe quantum dots were successfully synthesized in an aqueous solution using an internal doping method. The effects of ligand type, CuSe synthesis temperature, and heating time on Cu-doped ZnSe synthesis were systematically investigated. Of MPA, GSH, TGA, and NAC used as ligands, MPA was the optimal ligand as determined by PL spectrum analysis. In addition, the emission wavelength was found to depend on the synthesis temperature of the internal doping core of CuSe. As the temperature increased, the doping of Cu2+ was enhanced, and the emission wavelength band was redshifted; accordingly, the emission peaks moved from blue to green (up to 550 nm). Thus, the synthesis of Cu:ZnSe using internal doping in aqueous solutions is a potential method for ecomanufacturing of colortuned ZnSe quantum dots for display applications.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
- [Korean]
- Controlling the Heat Generation Capability of Iron Oxide-Base Nanoparticles
- Jin-sil Choi
- J Korean Powder Metall Inst. 2021;28(6):518-526. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.518

- 684 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF This review summarizes the recent progress in iron-oxide-based heat generators. Cancer treatment using magnetic nanoparticles as a heat generator, termed magnetic fluid hyperthermia, is a promising noninvasive approach that has gained significant interest. Most previous studies on improving the hyperthermia effect have focused on the construction of dopant-containing iron oxides. However, their applications in a clinical application can be limited due to extra dopants, and pure iron oxide is the only inorganic material approved by the Food and Drug Administration (FDA). Several factors that influence the heat generation capability of iron-oxide-based nanoparticles are summarized by reviewing recent studies on hyperthermia agents. Thus, our paper will provide the guideline for developing pure iron oxide-based heat generators with high heat dissipation capabilities.
- [Korean]
- Preparation of CoFe2O4 Nanoparticle Decorated on Electrospun Carbon Nanofiber Composite Electrodes for Supercapacitors
- Hyewon Hwang, Seoyeon Yuk, Minsik Jung, Dongju Lee
- J Korean Powder Metall Inst. 2021;28(6):470-477. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.470
- 743 View
- 4 Download
-
 Abstract
Abstract
 PDF
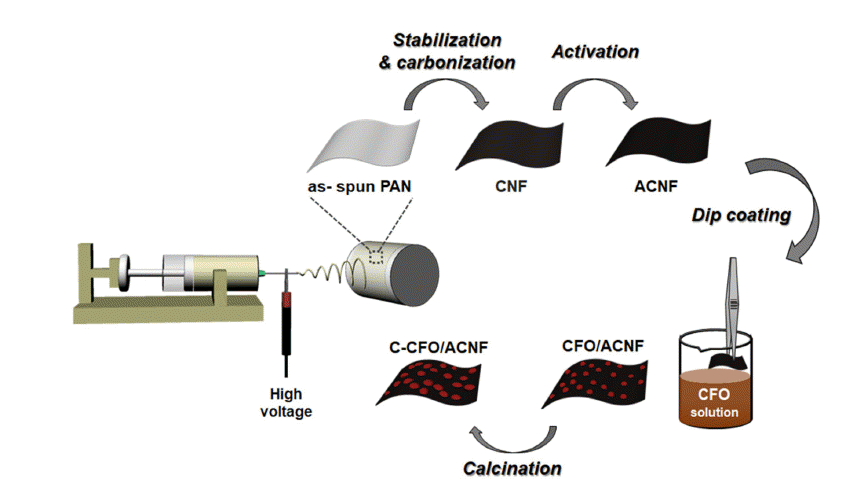
PDF Energy storage systems should address issues such as power fluctuations and rapid charge-discharge; to meet this requirement, CoFe2O4 (CFO) spinel nanoparticles with a suitable electrical conductivity and various redox states are synthesized and used as electrode materials for supercapacitors. In particular, CFO electrodes combined with carbon nanofibers (CNFs) can provide long-term cycling stability by fabricating binder-free three-dimensional electrodes. In this study, CFO-decorated CNFs are prepared by electrospinning and a low-cost hydrothermal method. The effects of heat treatment, such as the activation of CNFs (ACNFs) and calcination of CFO-decorated CNFs (C-CFO/ACNFs), are investigated. The C-CFO/ACNF electrode exhibits a high specific capacitance of 142.9 F/g at a scan rate of 5 mV/s and superior rate capability of 77.6% capacitance retention at a high scan rate of 500 mV/s. This electrode also achieves the lowest charge transfer resistance of 0.0063 Ω and excellent cycling stability (93.5% retention after 5,000 cycles) because of the improved ion conductivity by pathway formation and structural stability. The results of our work are expected to open a new route for manufacturing hybrid capacitor electrodes containing the C-CFO/ACNF electrode that can be easily prepared with a low-cost and simple process with enhanced electrochemical performance.
- [Korean]
- Synthesis and Application of Magnetoplasmonic Nanoparticles
- Sejeong Park, Siyeong Hwang, Seonghwan Jung, Juyong Gwak, Jaebeom Lee
- J Korean Powder Metall Inst. 2021;28(5):429-434. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.429

- 727 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF Magnetic nanoparticles have a significant impact on the development of basic sciences and nanomedical, electronic, optical, and biotech industries. The development of magnetic structures with size homogeneity, magnetization, and particle dispersibility due to high-quality process development can broaden their utilization for separation analysis, structural color optics using surface modification, and energy/catalysts. In addition, magnetic nanoparticles simultaneously exhibit two properties: magnetic and plasmon resonance, which can be self-assembled and can improve signal sensitivity through plasmon resonance. This paper reports typical examples of the synthesis and properties of various magnetic nanoparticles, especially magnetoplasmonic nanoparticles developed in our laboratory over the past decade, and their optical, electrochemical, energy/catalytic, and bio-applications. In addition, the future value of magnetoplasmonic nanoparticles can be reevaluated by comparing them with that reported in the literature.
- [Korean]
- Effects of Synthesis Conditions on Luminescence Characteristics of Glutathione Capped ZnSe Nano particles
- Geum Ji Back, Ha Yeon Song, Min Seo Lee, Hyun Seon Hong
- J Korean Powder Metall Inst. 2021;28(1):44-50. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.44
- 1,195 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
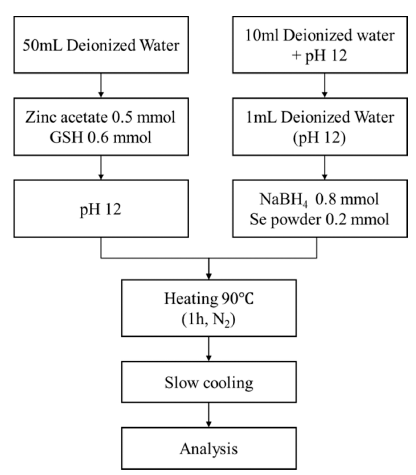
PDF Zinc selenide (ZnSe) nanoparticles were synthesized in aqueous solution using glutathione (GSH) as a ligand. The influence of the ligand content, reaction temperature, and hydroxyl ion concentration (pH) on the fabrication of the ZnSe particles was investigated. The optical properties of the synthesized ZnSe particles were characterized using various analytical techniques. The nanoparticles absorbed UV-vis light in the range of 350-400 nm, which is shorter than the absorption wavelength of bulk ZnSe particles (460 nm). The lowest ligand concentration for achieving good light absorption and emission properties was 0.6 mmol. The reaction temperature had an impact on the emission properties; photoluminescence spectroscopic analysis showed that the photo-discharge characteristics were greatly enhanced at high temperatures. These discharge characteristics were also affected by the hydroxyl ion concentration in solution; at pH 13, sound emission characteristics were observed, even at a low temperature of 25°C. The manufactured nanoparticles showed excellent light absorption and emission properties, suggesting the possibility of fabricating ZnSe QDs in aqueous solutions at low temperatures.
-
Citations
Citations to this article as recorded by- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
Geum Ji Back, Ha Yeon Song, Min Seo Lee, Jaesik Yoon, Hyun Seon Hong
Journal of Crystal Growth.2024; 626: 127475. CrossRef - Effect of UV Irradiation on Optical Properties of Water-Based Synthetic Zinc Selenide Quantum Dots
Geum Ji Back, Yu Jin Kang, I Ju Kang, Jeong Hyeon Lim, Hyun Seon Hong
Korean Journal of Metals and Materials.2022; 60(2): 160. CrossRef
- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
- [English]
- Fabrication and Characterization of Immiscible Fe-Cu Alloys using Electrical Explosion of Wire in Liquid
- Chu Dac Phuc, Nguyen Minh Thuyet, Jin-Chun Kim
- J Korean Powder Metall Inst. 2020;27(6):449-457. Published online December 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.6.449

- 1,567 View
- 12 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF Iron and copper are practically immiscible in the equilibrium state, even though their atomic radii are similar. As non-equilibrium solid solutions, the metastable Fe-Cu alloys can be synthesized using special methods, such as rapid quenching, vapor deposition, sputtering, ion-beam mixing, and mechanical alloying. The complexity of these methods (multiple steps, low productivity, high cost, and non-eco-friendliness) is a hinderance for their industrial applications. Electrical explosion of wire (EEW) is a well-known and effective method for the synthesis of metallic and alloy nanoparticles, and fabrication using the EEW is a simple and economic process. Therefore, it can be potentially employed to circumvent this problem. In this work, we propose the synthesis of Fe-Cu nanoparticles using EEW in a suitable solution. The powder shape, size distribution, and alloying state are analyzed and discussed according to the conditions of the EEW.
-
Citations
Citations to this article as recorded by- Scaling up plasma-derived metallic nanoalloys: A comprehensive review of production bottlenecks, manufacturing readiness, and AI-driven pathways to viability
Hugues Nkomba Museba, BongJu Lee
Journal of Alloys and Compounds.2026; 1054: 185926. CrossRef - Identification of the reconstruction induced high-entropy spinel oxide nanosheets for boosting alkaline water oxygen evolution
Xuexue Wang, Runqing Lu, Shanhe Gong, Shaokang Yang, Wenbo Wang, Zhongti Sun, Xiaozhen Zhang, Jun Liu, Xiaomeng Lv
Chemical Engineering Journal.2025; 503: 158488. CrossRef - Trends in bimetallic nanomaterials and methods for the removal of p-nitrophenol and its derivatives from wastewater
M. S. Qatan, F. Arshad, M. Miskam, G. A. Naikoo
International Journal of Environmental Science and Technology.2024; 21(5): 5247. CrossRef - Control of cluster coalescence during formation of bimetallic nanoparticles and nanoalloys obtained via electric explosion of two wires
K.V. Suliz, A.Yu. Kolosov, V.S. Myasnichenko, N.I. Nepsha, N.Yu. Sdobnyakov, A.V. Pervikov
Advanced Powder Technology.2022; 33(3): 103518. CrossRef
- Scaling up plasma-derived metallic nanoalloys: A comprehensive review of production bottlenecks, manufacturing readiness, and AI-driven pathways to viability
- [Korean]
- Synthesis and Nucleation Behavior of MoO3 Nano Particles with Concentration of Precursors
- Seyoung Lee, Namhun Kwon, Jaeseok Roh, Kun-Jae Lee
- J Korean Powder Metall Inst. 2020;27(5):394-400. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.394

- 1,531 View
- 8 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Molybdenum trioxide (MoO3) is used in various applications including sensors, photocatalysts, and batteries owing to its excellent ionic conductivity and thermal properties. It can also be used as a precursor in the hydrogen reduction process to obtain molybdenum metals. Control of the parameters governing the MoO3 synthesis process is extremely important because the size and shape of MoO3 in the reduction process affect the shape, size, and crystallization of Mo metal. In this study, we fabricated MoO3 nanoparticles using a solution combustion synthesis (SCS) method that utilizes an organic additive, thereby controlling their morphology. The nucleation behavior and particle morphology were confirmed using ultraviolet-visible spectroscopy (UV-vis) and field emission scanning electron microscopy (FE-SEM). The concentration of the precursor (ammonium heptamolybdate tetrahydrate) was adjusted to be 0.1, 0.2, and 0.4 M. Depending on this concentration, different nucleation rates were obtained, thereby resulting in different particle morphologies.
-
Citations
Citations to this article as recorded by- Characterization of Compacted and Pressureless Sintered Parts for Molybdenum Oxide Powder according to Hydrogen Reduction Temperature
Jong Hoon Lee, Kun-Jae Lee
Journal of Powder Materials.2024; 31(4): 336. CrossRef
- Characterization of Compacted and Pressureless Sintered Parts for Molybdenum Oxide Powder according to Hydrogen Reduction Temperature
- [Korean]
- Influence of Reducing Agents and Additives on the Synthesis of ZnSe Nanoparticles
- Geum Ji Back, Da Gyeong Lee, Min Seo Lee, Ha Yeon Song, Hyun Seon Hong
- J Korean Powder Metall Inst. 2020;27(3):233-240. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.233
- 625 View
- 1 Download
-
 Abstract
Abstract
 PDF
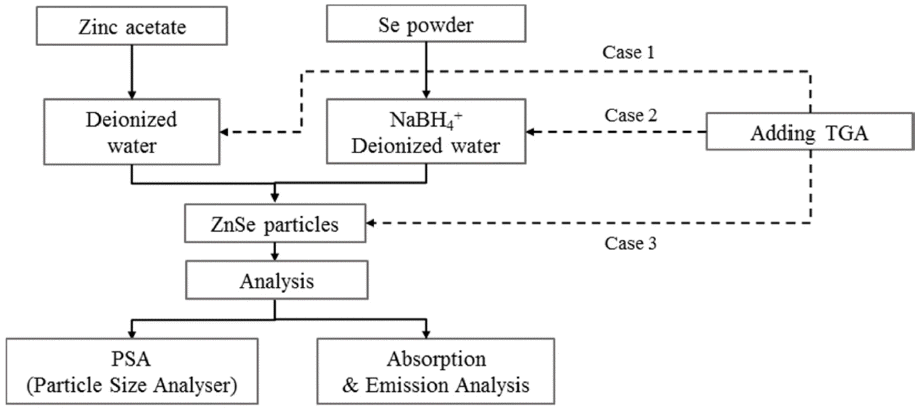
PDF Nano-sized ZnSe particles are successfully synthesized in an aqueous solution at room temperature using sodium borohydride (NaBH4) and thioglycolic acid (TGA) as the reducing agent and stabilizer, respectively. The effects of the mass ratio of the reducing agent to Se, stabilizer concentration, and stirring time on the synthesis of the ZnSe nanoparticles are evaluated. The light absorption/emission properties of the synthesized nanoparticles are characterized using ultraviolet-visible (UV-vis) spectroscopy, photoluminescence (PL) spectroscopy, and particle size analyzer (PSA) techniques. At least one mass ratio (NaBH4/Se) of the reducing agent should be added to produce ZnSe nanoparticles finer than 10 nm and to absorb UV–vis light shorter than the ZnSe bulk absorption wavelength of 460 nm. As the ratio of the reducing agent increases, the absorption wavelengths in the UV-vis curves are blue-shifted. Stirring in the atmosphere acts as a deterrent to the reduction reaction and formation of nanoparticles, but if not stirred in the atmosphere, the result is on par with synthesis in a nitrogen atmosphere. The stabilizer, TGA, has an impact on the Zn precursor synthesis. The fabricated nanoparticles exhibit excellent photo-absorption/discharge characteristics, suggesting that ZnSe nanoparticles can be alloyed without the need for organic solutions or high-temperature environments.
- [Korean]
- Fluorescent Nanoparticles: Synthesis and Applications
- Y. K. Kim, B. K. Song, J. G. Lee, Y. K. Baek
- J Korean Powder Metall Inst. 2020;27(2):154-163. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.154
- 2,905 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
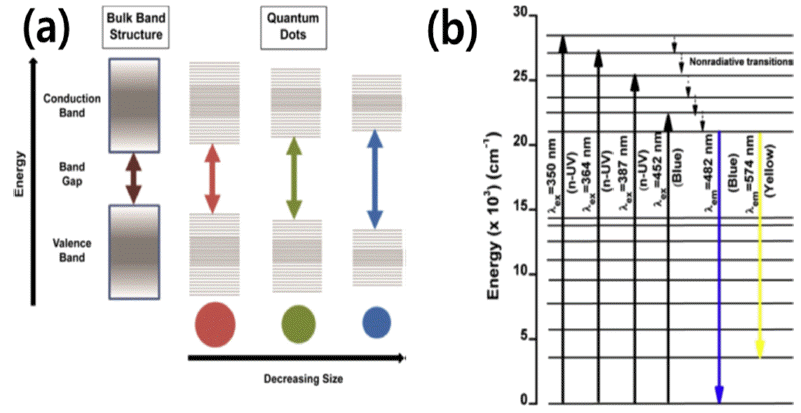
PDF Fluorescent nanoparticles are characterized by their unique properties such as luminescence, optical transparency, and sensitivity to various chemical environments. For example, semiconductor nanocrystals (quantum dots), which are nanophosphors doped with transition metal or rare earth ions, can be classified as fluorescent nanoparticles. Tuning their optical and physico-chemical properties can be carried out by considering and taking advantage of nanoscale effects. For instance, quantum confinement causes a much higher fluorescence with nanoparticles than with their bulk counterparts. Recently, various types of fluorescent nanoparticles have been synthesized to extend their applications to other fields. In this study, State-of-the-art fluorescent nanoparticles are reviewed with emphasis on their analytical and anti-counterfeiting applications and synthesis processes. Moreover, the fundamental principles behind the exceptional properties of fluorescent nanoparticles are discussed.
-
Citations
Citations to this article as recorded by- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
Ji-Won Heo, A-Young Sung
Korean Journal of Materials Research.2022; 32(4): 193. CrossRef
- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
- [Korean]
- Synthesis of the Multifunctional Core/Intermediate/Shell Nanoparticles: Tunable Magnetic and Photoluminescence Properties
- Mun-Kyoung Kim, Seyun Kim, Kyoung-Seok Moon, Weon Ho Shin, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2019;26(6):463-470. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.463
- 739 View
- 3 Download
-
 Abstract
Abstract
 PDF
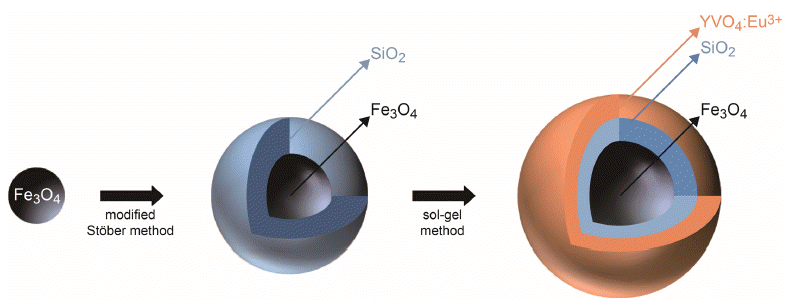
PDF Fe3O4/SiO2/YVO4:Eu3+ multifunctional nanoparticles are successfully synthesized by facile stepwise sol-gel processes. The multifunctional nanoparticles show a spherical shape with narrow size distribution (approximately 40 nm) and the phosphor shells are well crystallized. The Eu3+ shows strong photoluminescence (red emission at 619 nm, absorbance at 290 nm) due to an effective energy transfer from the vanadate group to Eu. Core-shell structured multifunctional nanoparticles have superparamagnetic properties at 300 K. Furthermore, the core-shell nanoparticles have a quick response time for the external magnetic field. These results suggest that the photoluminescence and magnetic properties could be easily tuned by either varying the number of coating processes or changing the phosphor elements. The nanoparticles may have potential applications for appropriate fields such as laser systems, optical amplifiers, security systems, and drug delivery materials.
- [Korean]
- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Jae-Hyun Yoo, Myeong-Jun Ji, Woo-Young Park, Young-In Lee
- J Korean Powder Metall Inst. 2019;26(3):237-242. Published online June 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.3.237
- 822 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, ultrasonic spray pyrolysis combined with salt-assisted decomposition, a process that adds sodium nitrate (NaNO3) into a titanium precursor solution, is used to synthesize nanosized titanium dioxide (TiO2) particles. The added NaNO3 prevents the agglomeration of the primary nanoparticles in the pyrolysis process. The nanoparticles are obtained after a washing process, removing NaNO3 and NaF from the secondary particles, which consist of the salts and TiO2 nanoparticles. The effects of pyrolysis temperature on the size, crystallographic characteristics, and bandgap energy of the synthesized nanoparticles are systematically investigated. The synthesized TiO2 nanoparticles have a size of approximately 2–10 nm a bandgap energy of 3.1–3.25 eV, depending on the synthetic temperature. These differences in properties affect the photocatalytic activities of the synthesized TiO2 nanoparticles.
-
Citations
Citations to this article as recorded by- Microstructure and Sintering Behavior of Fine Tungsten Powders Synthesized by Ultrasonic Spray Pyrolysis
Hyeonhui Jo, Jeong Hyun Kim, Young-In Lee, Young-Keun Jeong, Sung-Tag Oh
Korean Journal of Metals and Materials.2021; 59(5): 289. CrossRef
- Microstructure and Sintering Behavior of Fine Tungsten Powders Synthesized by Ultrasonic Spray Pyrolysis
- [Korean]
- Synthesis and Optical Property of TiO2 Nanoparticles Using a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Myeong-Jun Ji, Woo-Young Park, Jae-Hyun Yoo, Young-In Lee
- J Korean Powder Metall Inst. 2019;26(1):34-39. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.34
- 823 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
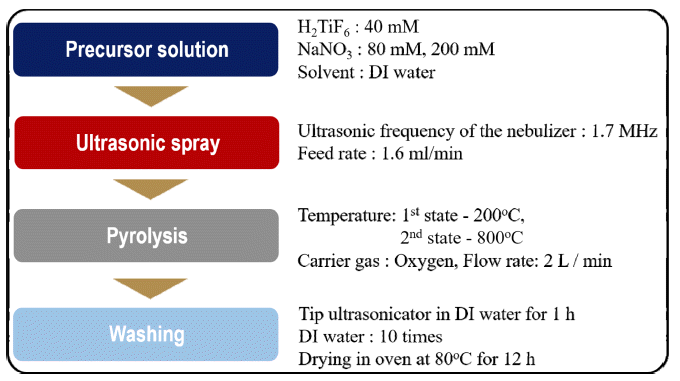
PDF Current synthesis processes for titanium dioxide (TiO2) nanoparticles require expensive precursors or templates as well as complex steps and long reaction times. In addition, these processes produce highly agglomerated nanoparticles. In this study, we demonstrate a simple and continuous approach to synthesize TiO2 nanoparticles by a salt-assisted ultrasonic spray pyrolysis method. We also investigate the effect of salt content in a precursor solution on the morphology and size of synthesized products. The synthesized TiO2 nanoparticles are systematically characterized by X-ray diffraction, transmission electron micrograph, and UV-Vis spectroscopy. These nanoparticles appear to have a single anatase phase and a uniform particle-size distribution with an average particle size of approximately 10 nm. By extrapolating the plots of the transformed Kubelka-Munk function versus the absorbed light energy, we determine that the energy band gap of the synthesized TiO2 nanoparticles is 3.25 eV.
-
Citations
Citations to this article as recorded by- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
Jae-Hyun Yoo, Myeong-Jun Ji, Woo-Young Park, Young-In Lee
Journal of Korean Powder Metallurgy Institute.2019; 26(3): 237. CrossRef
- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
- [Korean]
- Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
- Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2018;25(5):420-425. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.420

- 923 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Core-shell structured nanoparticles are garnering attention because these nanoparticles are expected to have a wide range of applications. The objective of the present study is to improve the coating efficiency of gold shell formed on the surface of silica nanoparticles for SiO2@Au core-shell structure. For the efficient coating of gold shell, we attempt an in-situ synthesis method such that the nuclei of the gold nanoparticles are generated and grown on the surface of silica nanoparticles. This method can effectively form a gold shell as compared to the conventional method of attaching gold nanoparticles to silica particles. It is considered possible to form a dense gold shell because the problems caused by electrostatic repulsion between the gold nanoparticles in the conventional method are eliminated.
- [Korean]
- Synthesis of Nanopowders by Hydrothermal Method and their Application to Dye-sentisized Solar Cell Materials
- JinYoung Lim, Jeongseok Ahn, Jung-Ho Ahn
- J Korean Powder Metall Inst. 2018;25(4):309-315. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.309
- 679 View
- 2 Download
-
 Abstract
Abstract
 PDF
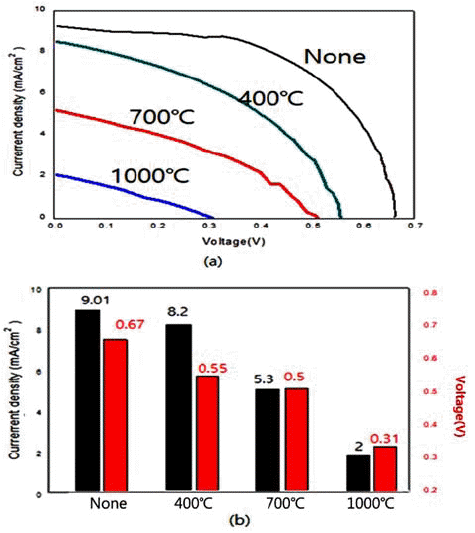
PDF In the present work, we synthesize nano-sized ZnO, SnO2, and TiO2 powders by hydrothermal reaction using metal chlorides. We also examine the energy-storage characteristics of the resulting materials to evaluate the potential application of these powders to dye-sensitized solar cells. The control of processing parameters such as pressure, temperature, and the concentration of aqueous solution results in the formation of a variety of powder morphologies with different sizes. Nano-rod, nano-flower, and spherical powders are easily formed with the present method. Heat treatment after the hydrothermal reaction usually increases the size of the powder. At temperatures above 1000°C, a complete collapse of the shape occurs. With regard to the capacity of DSSC materials, the hydrothermally synthesized TiO2 results in the highest current density of 9.1 mA/cm2 among the examined oxides. This is attributed to the fine particle size and morphology with large specific surface area.
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
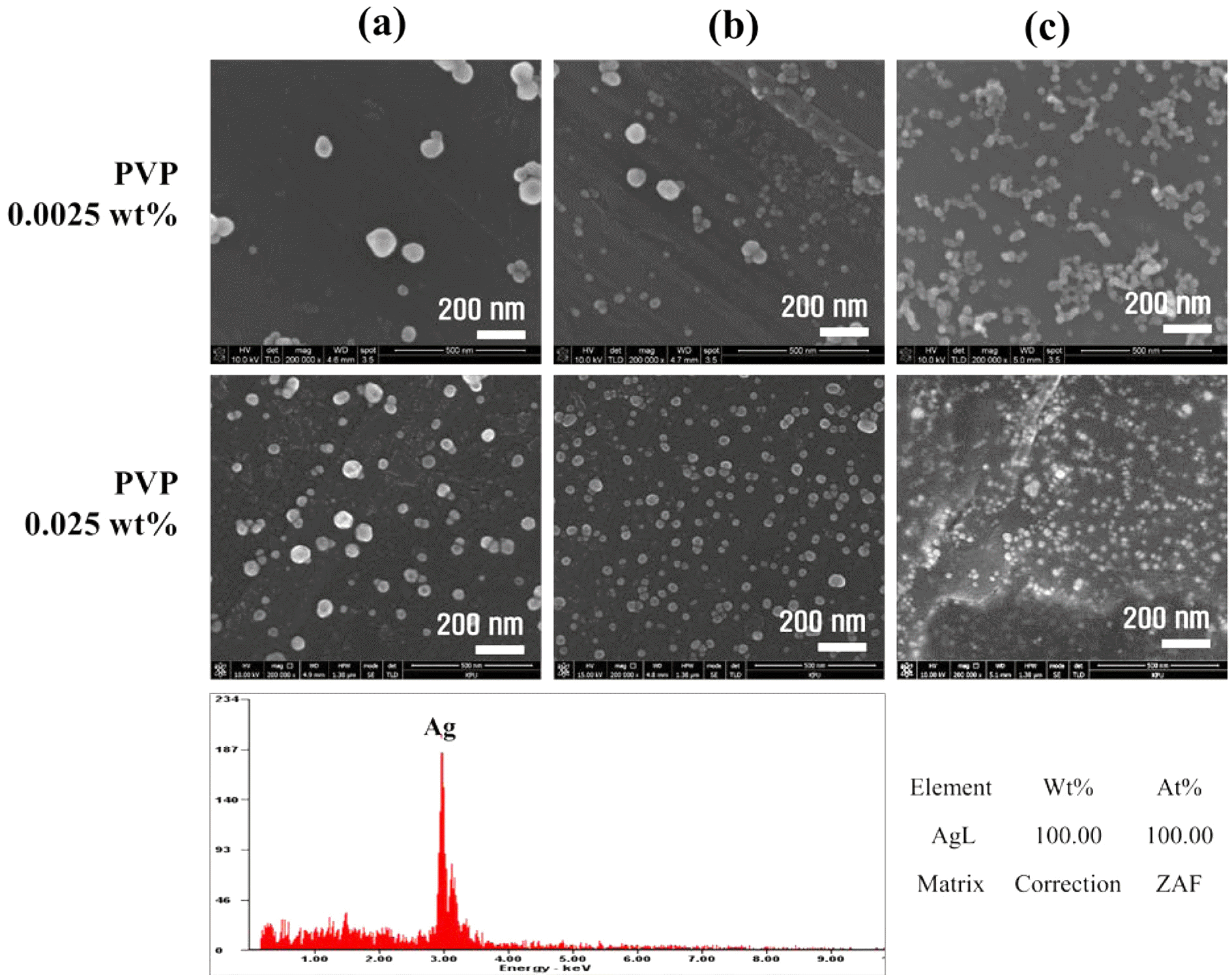
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 875 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- Technology Trend of Luminescent Nanomaterials
- Hyewon Jeong, Jae Sung Son
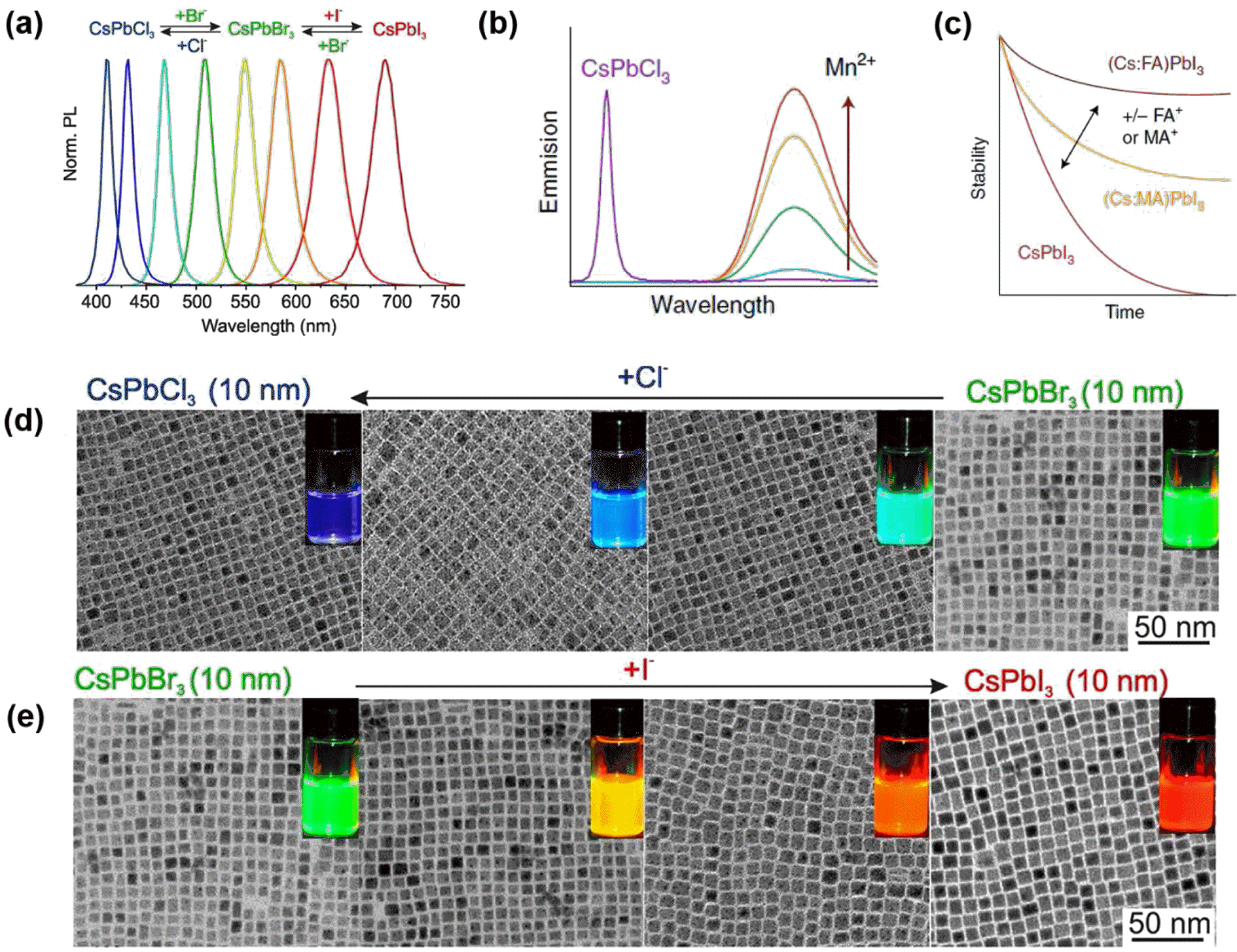
- J Korean Powder Metall Inst. 2018;25(2):170-177. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.170
- 1,093 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Colloidally synthesized luminescent nanocrystals (NCs) have attracted tremendous attention due to their unique nanoscale optical and electronic properties. The emission properties of these NCs can be precisely tuned by controlling their size, shape, and composition as well as by introducing appropriate dopant impurities. Nowadays, these NCs are actively utilized for various applications such as optoelectronic devices including light emitting diodes (LEDs), lasers, and solar cells, and bio-medical applications such as imaging agents and bio-sensors. In this review, we classify luminescent nanomaterials into quantum dots (QDs), upconversion nanoparticles (UCNPs), and perovskite NCs and present their intrinsic emission mechanism. Furthermore, the recently emerging issues of efficiency, toxicity, and durability in these materials are discussed for better understanding of industry demands. As well, the future outlook will be offered for researchers to guide the direction of future research.
-
Citations
Citations to this article as recorded by- A Structural Relationship between University Dance Students’ Emotional Regulation, Emotion Response, and Engagement in Classes
Jinhee Gong
The Journal of Korean Institute of Information Technology.2020; 18(4): 121. CrossRef
- A Structural Relationship between University Dance Students’ Emotional Regulation, Emotion Response, and Engagement in Classes
- [Korean]
- Synthesis and Characterization of Core-Shell Silica-Phosphor Nanoparticles
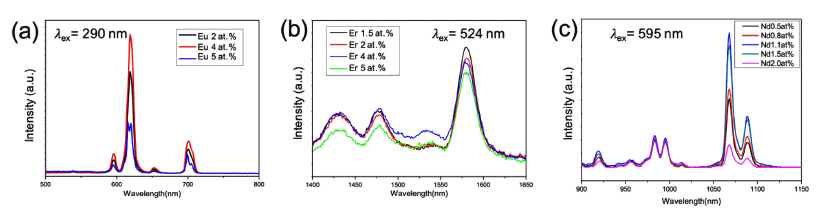
via Sol-Gel Process - Weon Ho Shin, Seyun Kim, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2018;25(1):12-18. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.12
- 1,000 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Cost-effective functional phosphor nanoparticles are prepared by introducing low-cost SiO2 spheres to rareearth phosphor (YVO4:Eu3+, YVO4:Er3+, and YVO4:Nd3+) shells using a sol-gel synthetic method. These functional nanoparticles are characterized by X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, and general photoluminescence spectra. The SiO2 sphere occupying the interior of the conventional phosphor is advantageous in significantly reducing the cost of expensive rare-earth phosphor nanoparticles. The sol-gel process facilitates the core–shell structure formation; the rare-earth shell phosphor has strong interactions with chelating agents on the surfaces of SiO2 nanoparticles and thus forms layers of several nanometers in thickness. The photoluminescence wavelength is simply tuned by replacing the active materials of Eu3+, Er3+, and Nd3+. Moreover, the photoluminescent properties of the core–shell nanoparticles can be optimized by manipulating the specific contents of active materials in the phosphors. Our simple approach substitutes low-cost SiO2 for expensive rare-earth-based phosphor materials to realize cost-effective phosphor nanoparticles for various applications.
-
Citations
Citations to this article as recorded by- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
Woo Hyeong Sim, Seyun Kim, Weon Ho Shin, Hyung Mo Jeong
Korean Journal of Metals and Materials.2020; 58(2): 137. CrossRef
- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
- [English]
- The Synthesis Method of Tin Dioxide Nanoparticles by Plasma-Assisted Electrolysis Process and Gas Sensing Property
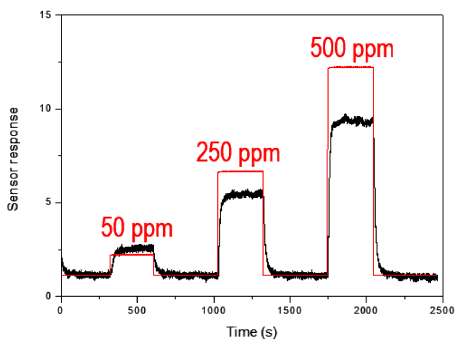
- Tae Hyung Kim, Yoseb Song, Chan-Gi Lee, Yong-Ho Choa
- J Korean Powder Metall Inst. 2017;24(5):351-356. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.351
- 1,519 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Tin dioxide nanoparticles are prepared using a newly developed synthesis method of plasma-assisted electrolysis. A high voltage is applied to the tin metal plate to apply a high pressure and temperature to the synthesized oxide layer on the metal surface, producing nanoparticles in a low concentration of sulfuric acid. The particle size, morphology, and size distribution is controlled by the concentration of electrolytes and frequency of the power supply. The as-prepared powder of tin dioxide nanoparticles is used to fabricate a gas sensor to investigate the potential application. The particle-based gas sensor exhibits a short response and recovery time. There is sensitivity to the reduction gas for the gas flowing at rates of 50, 250, and 500 ppm of H2S gas.
-
Citations
Citations to this article as recorded by- Effects of porosity and particle size on the gas sensing properties of SnO2 films
Min Ah Han, Hyun-Jong Kim, Hee Chul Lee, Jin-Seong Park, Ho-Nyun Lee
Applied Surface Science.2019; 481: 133. CrossRef
- Effects of porosity and particle size on the gas sensing properties of SnO2 films
- [Korean]
- Synthesis and Optical Property of BaTiO3 Nanoparticles Using a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Young Hwangbo, Young-In Lee
- J Korean Powder Metall Inst. 2017;24(4):326-331. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.326
- 896 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The structural formation of inorganic nanoparticles dispersed in polymer matrices is a key technology for producing advanced nanocomposites with a unique combination of optical, electrical, and mechanical properties. Barium titanate (BaTiO3) nanoparticles are attractive for increasing the refractive index and dielectric constant of polymer nanocomposites. Current synthesis processes for BaTiO3 nanoparticles require expensive precursors or organic solvents, complicated steps, and long reaction times. In this study, we demonstrate a simple and continuous approach for synthesizing BaTiO3 nanoparticles based on a salt-assisted ultrasonic spray pyrolysis method. This process allows the synthesis of BaTiO3 nanoparticles with diameters of 20-50 nm and a highly crystalline tetragonal structure. The optical properties and photocatalytic activities of the nanoparticles show that they are suitable for use as fillers in various nanocomposites.
-
Citations
Citations to this article as recorded by- Sr doping effects on La(1-x)SrxMnO3-BaTiO3 nanocomposites: A comprehensive analysis of structural, optical, magnetic, and dielectric properties
Milad Karamzadeh-Jahromi, Morteza Izadifard, Mohammad Ebrahim Ghazi
Journal of Alloys and Compounds.2024; 1006: 176272. CrossRef
- Sr doping effects on La(1-x)SrxMnO3-BaTiO3 nanocomposites: A comprehensive analysis of structural, optical, magnetic, and dielectric properties
- [Korean]
- Recovery and Synthesis of Silver Nanoparticles from Leaching Solution of LTCC Electrode By-Products
- Juyeon Yoo, Yubin Kang, Jinju Park, Hojin Ryu, Jin-Ho Yoon, Kun-Jae Lee
- J Korean Powder Metall Inst. 2017;24(4):315-320. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.315

- 502 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF There has been much interest in recycling electronic wastes in order to mitigate environmental problems and to recover the large amount of constituent metals. Silver recovery from electronic waste is extensively studied because of environmental and economic benefits and the use of silver in fabricating nanodevices. Hydrometallurgical processing is often used for silver recovery because it has the advantages of low cost and ease of control. Research on synthesis recovered silver into nanoparticles is needed for application to transistors and solar cells. In this study, silver is selectively recovered from the by-product of electrodes. Silver precursors are prepared using the dissolution characteristics of the leaching solution. In the liquid reduction process, silver nanoparticles are synthesized under various surfactant conditions and then analyzed. The purity of the recovered silver is 99.24%, and the average particle size of the silver nanoparticles is 68 nm.
- [English]
- Magnetically Driven Assemblies of γ-Fe3O4 Nanoparticles into Well-Ordered Permanent Structures
- Myunghwan Byun
- J Korean Powder Metall Inst. 2017;24(3):229-234. Published online June 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.3.229
- 845 View
- 1 Download
-
 Abstract
Abstract
 PDF
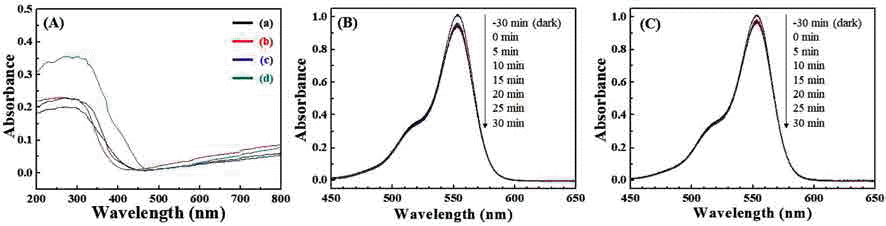
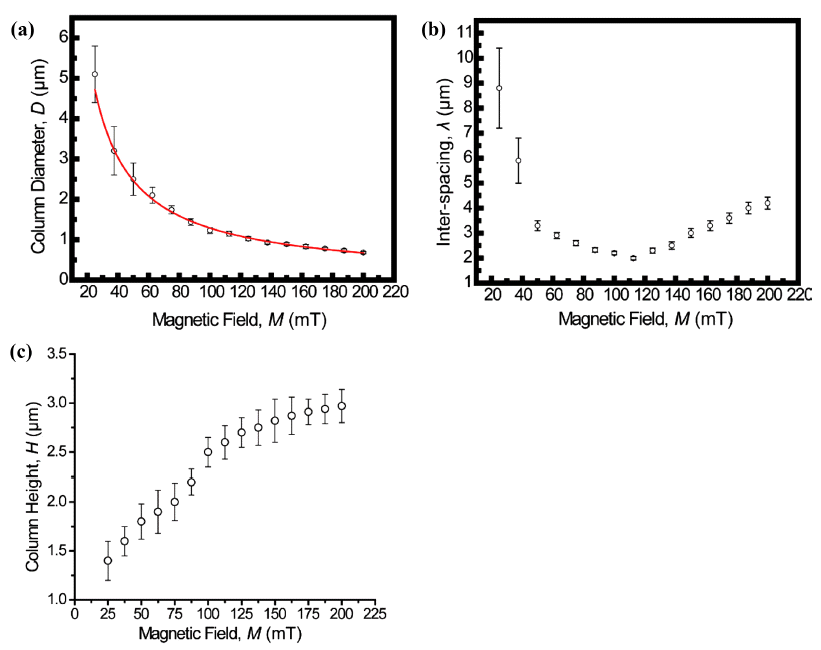
PDF We report on a simple and robust route to the spontaneous assembly of well-ordered magnetic nanoparticle superstructures by irreversible evaporation of a sessile single droplet of a mixture of a ferrofluid (FF) and a nonmagnetic fluid (NF). The resulting assembled superstructures are seen to form well-packed, vertically arranged columns with diameters of 5~0.7 μm, interparticle spacings of 9~2 μm, and heights of 1.3~3 μm. The assembled superstructures are strongly dependent on both the magnitude of magnetic field and the mixing ratio of the mixture. As the magnitude of the externally applied magnetic field and the mixing ratio of the mixture increase gradually, the size and interspacing of the magnetic nanoparticle aggregations decrease. Without an externally applied magnetic field, featureless patterns are observed for the γ-Fe3O4 nanoparticle aggregations. The proposed approach may lead to a versatile, cost-effective, fast, and scalable fabrication process based on the field-induced self-assembly of magnetic nanoparticles.
- [Korean]
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,029 View
- 8 Download
-
 Abstract
Abstract
 PDF
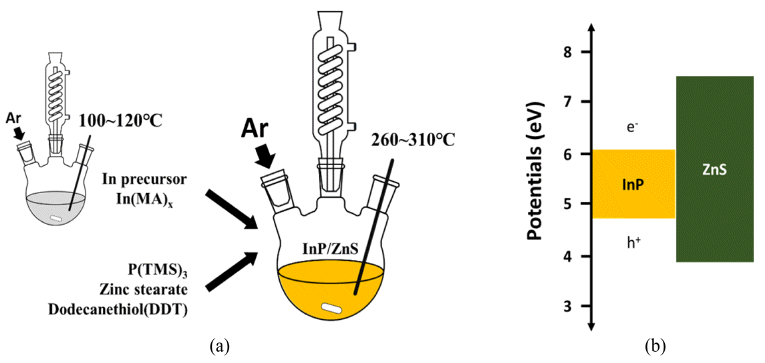
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
- Effect of Reaction Parameters on Silica Nanoparticles Synthesized by Sol-gel Method
- Young-Hyun Lim, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2016;23(6):442-446. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.442
- 3,027 View
- 47 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
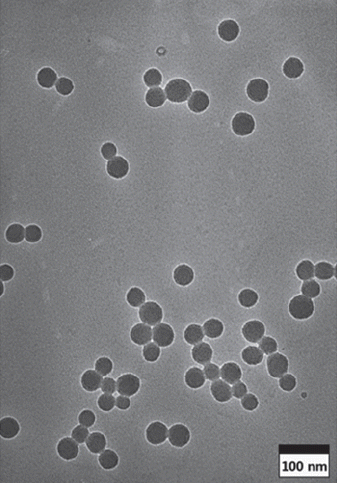
PDF The sol-gel method is the simplest method for synthesizing monodispersed silica particles. The purpose of this study is to synthesize uniform, monodisperse spherical silica nanoparticles using tetraethylorthosilicate (TEOS) as the silica precursor, ethanol, and deionized water in the presence of ammonia as a catalyst. The reaction time and temperature and the concentration of the reactants are controlled to investigate the effect of the reaction parameters on the size of the synthesized particles. The size and morphology of the obtained silica particles are investigated using transmission electron microscopy and particle size analysis. The results show that monodispersed silica particles over a size range of 54-504 nm are successfully synthesized by the sol-gel method without using any additional process. The nanosized silica particles can be synthesized at higher TEOS/H2O ratios, lower ammonia concentrations, and especially, higher reaction temperatures.
-
Citations
Citations to this article as recorded by- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
Mustapha Sulaiman, Naseer Inuwa Durumin Iya, Mamudu Aliyu
FUDMA JOURNAL OF SCIENCES.2024; 8(3): 222. CrossRef - Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 420. CrossRef
- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
- [Korean]
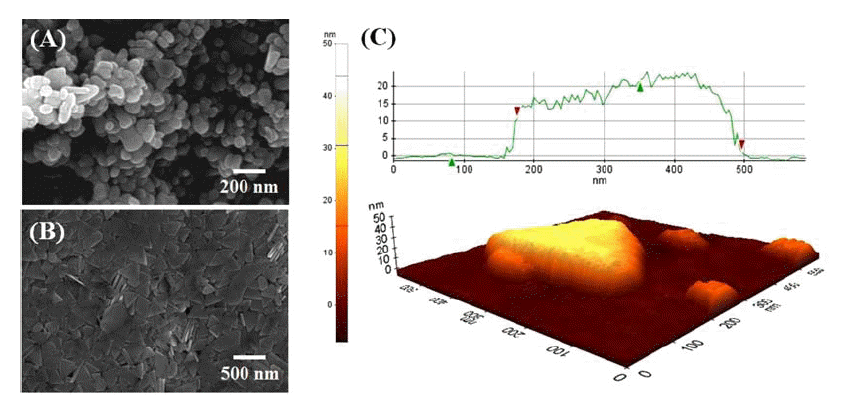
- Investigation on Microstructure and Electrical Properties of Silver Conductive Features Using a Powder Composed of Silver nanoparticles and Nanoplatelets
- Yong-Sung Goo, Yong-Ho Choa, Young Hwangbo, Young-In Lee
- J Korean Powder Metall Inst. 2016;23(5):358-363. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.358
- 558 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Noncontact direct-printed conductive silver patterns with an enhanced electrical resistivity are fabricated using a silver ink with a mixture of silver nanoparticles and nanoplates. The microstructure and electrical resistivity of the silver pattern are systematically investigated as a function of the mixing ratio of the nanoparticles and nanoplates. The pattern, which is fabricated using a mixture with a mixing ratio of 3(nanoparticles):7(nanoplates) and sintered at 200°C shows a highly dense and well-sintered microstructure and has a resistivity of 7.60 μΩ·cm. This originates a mutual synergistic effect through a combination of the sinterability of the nanoparticles and the packing ability of the nanoplates. This is a conductive material that can be used to fabricate noncontact direct-printed conductive patterns with excellent electrical conductivity for various flexible electronics applications, including solar cells, displays, RFIDs, and sensors.
- [Korean]
- Fabrication and Characterization of Ag Particles by Polyol Process and Wet Chemical Process
- Juyeon Yoo, Hyosung Jang, Kun-Jae Lee
- J Korean Powder Metall Inst. 2016;23(4):297-302. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.297
- 804 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Ag nanoparticles are extensively studied and utilized due to their excellent catalysis, antibiosis and optical properties. They can be easily synthesized by chemical reduction methods and it is possible to prepare particles of uniform size and high purity. These methods are divided into vapor methods and liquid phase reduction methods. In the present study, Ag particles are prepared and analyzed through two chemical reduction methods using solvents containing a silver nitrate precursor. When Ag ions are reduced using a reductant in the aqueous solution, it is possible to control the Ag particle size by controlling the formic acid ratio. In addition, in the Polyol process, Ag nanoparticles prepared at various temperatures and reaction time conditions have multiple twinned and anisotropic structures, and the particle size variation can be confirmed using field emissions scanning electron microscopy and by analyzing the UV-vis spectrum.
-
Citations
Citations to this article as recorded by- Recovery and Synthesis of Silver Nanoparticles from Leaching Solution of LTCC Electrode By-Products
Juyeon Yoo, Yubin Kang, Jinju Park, Hojin Ryu, Jin-Ho Yoon, Kun-Jae Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(4): 315. CrossRef
- Recovery and Synthesis of Silver Nanoparticles from Leaching Solution of LTCC Electrode By-Products
- [Korean]
- Synthesis of Copper Nanoparticles by a Chemical Reduction Method
- Min Woo Choi, Min Hwan Bae, Jung-Ho Ahn
- J Korean Powder Metall Inst. 2016;23(3):228-234. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.228
- 2,137 View
- 42 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Copper nanoparticles attract much attention as substitutes of noble metals such as silver and can help reduce the manufacturing cost of electronic products due to their lower cost and good conductivity. In the present work, the chemical reduction is examined to optimize the synthesis of nano-sized copper particles from copper sulfate. Sodium borohydride and ascorbic acid are used as reducing and antioxidant agents, respectively. Polyethylene glycol (PEG) is used as a size-control and capping agent. An appropriate dose of PEG inhibits the abnormal growth of copper nanoparticles, maintaining chemical stability. The addition of ascorbic acid prevents the oxidation of nanoparticles during synthesis and storage. Transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR) are used to investigate the size of the synthesized nanoparticles and the coordination between copper nanoparticles and PEG. For chemical reduction, copper nanoparticles less than 100 nm in size without oxidized layers are successfully obtained by the present method.
-
Citations
Citations to this article as recorded by- Correlation between electrical conductivity and antibacterial activity of chitosan-stabilized copper and silver nanoparticles
C.Raja Mohan, Ruckmani Kandasamy, J. Kabiriyel
Carbohydrate Polymer Technologies and Applications.2024; 7: 100503. CrossRef - Green Synthesis and Characterization of Natural Magnetic Particles/Chitosan Composite Material Impregnated with Copper Nanoparticles
Defia Indah Permatasari, Bambang Rusdiarso, Nuryono Nuryono
Solid State Phenomena.2022; 339: 19. CrossRef - Copper Nanoparticle(CuNP’s)Synthesis: A review of the various ways with Photocatalytic and Antibacterial Activity
Israfil Alam Tito, Sahab Uddin, Shafiul Islam, Snahasish Bhowmik
Oriental Journal Of Chemistry.2021; 37(5): 1030. CrossRef
- Correlation between electrical conductivity and antibacterial activity of chitosan-stabilized copper and silver nanoparticles
- [Korean]
- Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
- Hyeong-Chul Kim, Jae-Kil Han
- J Korean Powder Metall Inst. 2016;23(1):33-37. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.33

- 1,005 View
- 3 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF 10 wt.% and 20 wt.%Li-TiO2 composite powders are synthesized by a sol-gel method using titanium isopropoxide and Li2CO3 as precursors. The as-received amorphous 10 wt.%Li-TiO2 composite powders crystallize into the anatase-type crystal structure upon calcination at 450°C, which then changes to the rutile phase at 750°C. The asreceived 20 wt%Li-TiO2 composite powders, on the other hand, crystallize into the anatase-type structure. As the calcination temperature increases, the anatase TiO2 phase gets transformed to the LiTiO2 phase. The peaks for the samples obtained after calcination at 900°C mainly exhibit the LiTiO2 and Li2TiO3 phases. For a comparison of the photocatalytic activity, 10 wt.% and 20 wt.% Li-TiO2 composite powders calcined at 450°C, 600°C, and 750°C are used. The 20 wt.%Li-TiO2 composite powders calcined at 600°C show excellent efficiency for the removal of methylorange.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and characterization of nitrogen-doped TiO2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity
Yifan Zhang, Hye Mee Yang, Soo-Jin Park
Current Applied Physics.2018; 18(2): 163. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
- [Korean]
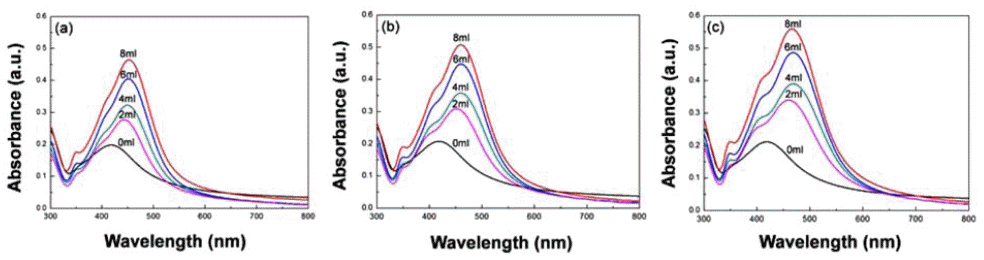
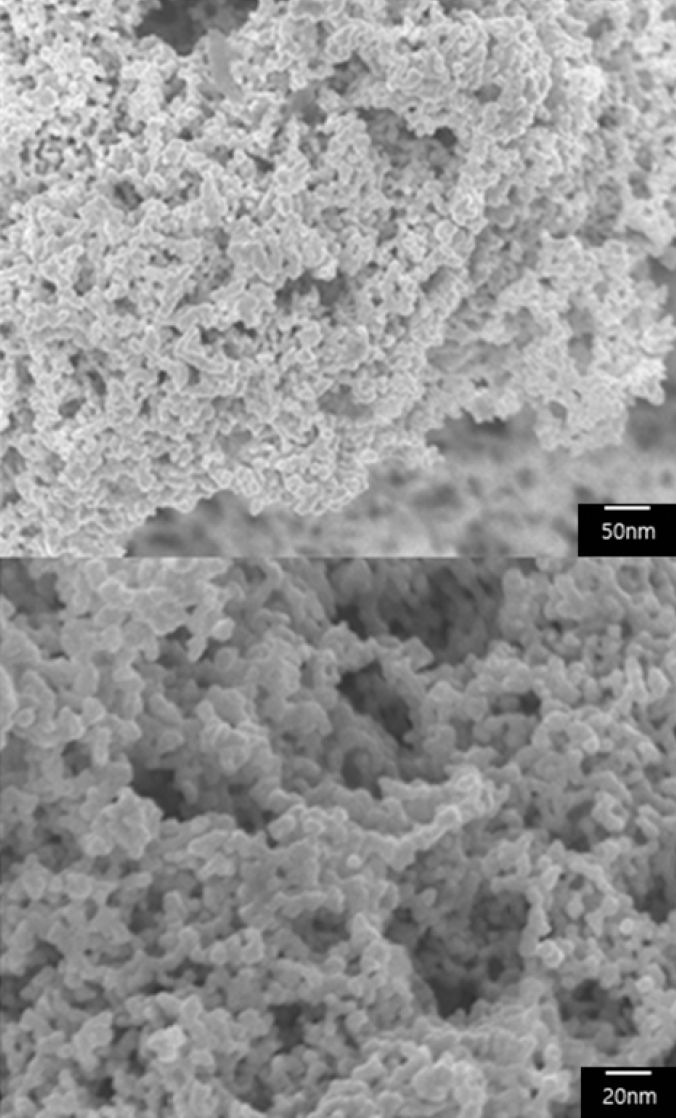
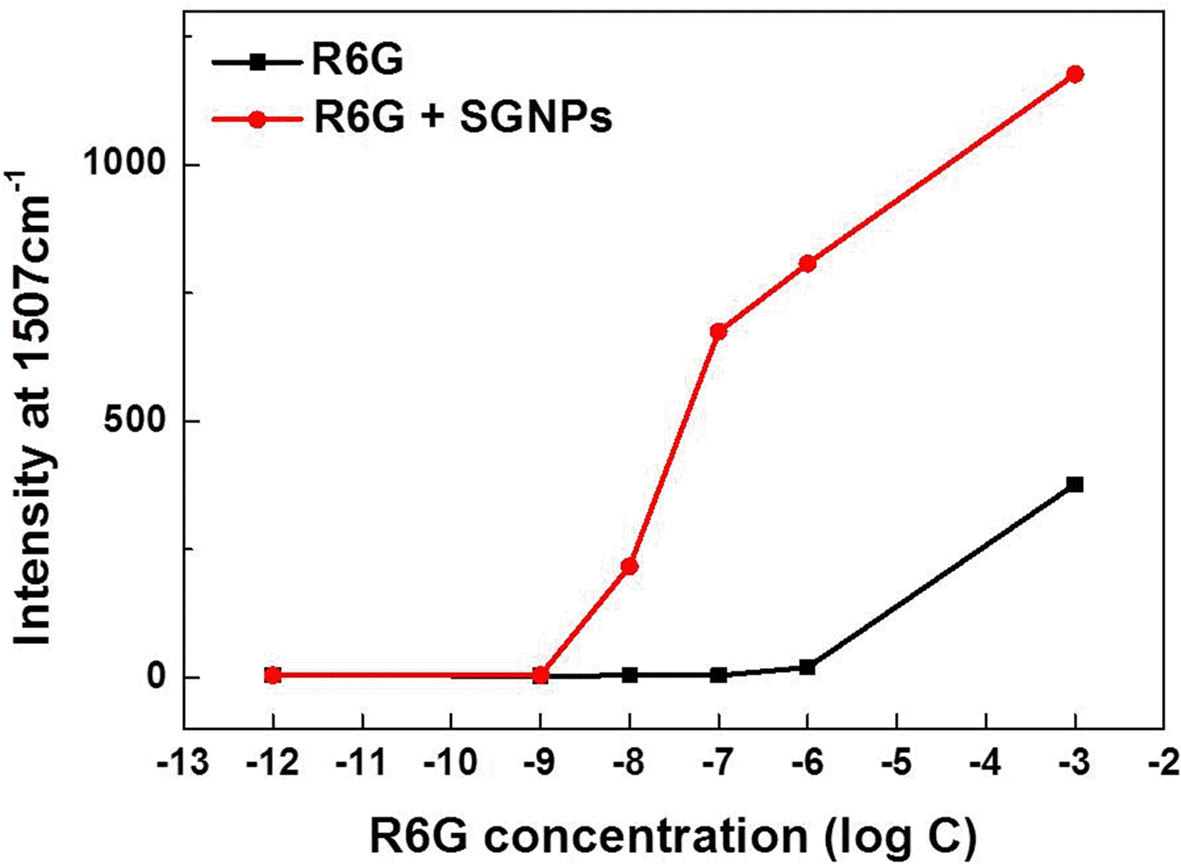
- Synthesis of Silica-Core Gold-Satellite Nanoparticles and Their Surface-enhanced Raman Scattering Based Sensing Application
- Hyun Ji Choi, Young-Kuk Kim, Seok-Young Yoon, Youn-Kyoung Baek
- J Korean Powder Metall Inst. 2014;21(6):441-446. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.441
- 1,485 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF In this study, we synthesize silica-core gold-satellite nanoparticles (SGNPs) for the surface-enhanced Raman scattering (SERS) based sensing applications. They consist of gold satellite nanoparticles (AuNPs) fixed on the silica core nanoparticles, which sizes of AuNPs can be tunned by varying the amount of reactants (growth solution and reducing agent). Their surface plasmon resonance (SPR) properties were characterized by using UV-vis spectroscopy, showing that the growth of AuNPs on silica cores leads to the light absorption in the longer wavelength region. Furthermore, the size increase of AuNPs exhibited the dramatic change in SERS activity due to the formation of hot spots. The optimized SGNPs showing enhancement factor~3.8×106 exhibited a detection limit of rhodamine 6G (R6G) as low as 10−8 M. These findings suggest the importance of size control of SGNPs and their SPR properties to develop highly efficient SERS sensors.
- [Korean]
- Synthesis of Highly Dispersible Metal Nanoparticles in P3HT:PCBM Layers and Their Effects on the Performance of Polymer Solar Cells
- Min-Ji Kim, Gyu-Chae Choi, Young-Kuk Kim, Yang-Do Kim, Youn-Kyoung Baek
- J Korean Powder Metall Inst. 2014;21(3):179-184. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.179

- 473 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF In this study, we prepare polymer solar cells incorporating organic ligand-modified Ag nanoparticles (OAgNPs) highly dispersed in the P3HT:PCBM layer. Ag nanoparticles decorated with water-dispersible ligands (WAgNPs) were also utilized as a control sample. The existence of the ligands on the Ag surface was confirmed by FTIR spectra. Metal nanoparticles with different surface chemistries exhibited different dispersion tendencies. O-AgNPs were highly dispersed even at high concentrations, whereas W-AgNPs exhibited significant aggregation in the polymer layer. Both dispersion and blending concentration of the Ag nanoparticles in P3HT:PCBM matrix had critical effects on the device performance as well as light absorption. The significant changes in short-circuit current density (JSC) of the solar cells seemed to be related to the change in the polymer morphology according to the concentration of AgNPs introduced. These findings suggested the importance of uniform dispersion of plasmonic metal nanoparticles and their blending concentration conditions in order to boost the solar cell performance.
TOP
 KPMI
KPMI


 First
First Prev
Prev


