Search
- Page Path
- HOME > Search
- [Korean]
- Effect of the Initial Porosity of Needle Coke-Pitch Carbonized Blocks on Impregnation-Related Physical Properties
- U-Sang Youn, Sang-Hye Lee, Jae-Seung Roh
- J Powder Mater. 2025;32(2):138-144. Published online April 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00038
- 719 View
- 14 Download
-
 Abstract
Abstract
 PDF
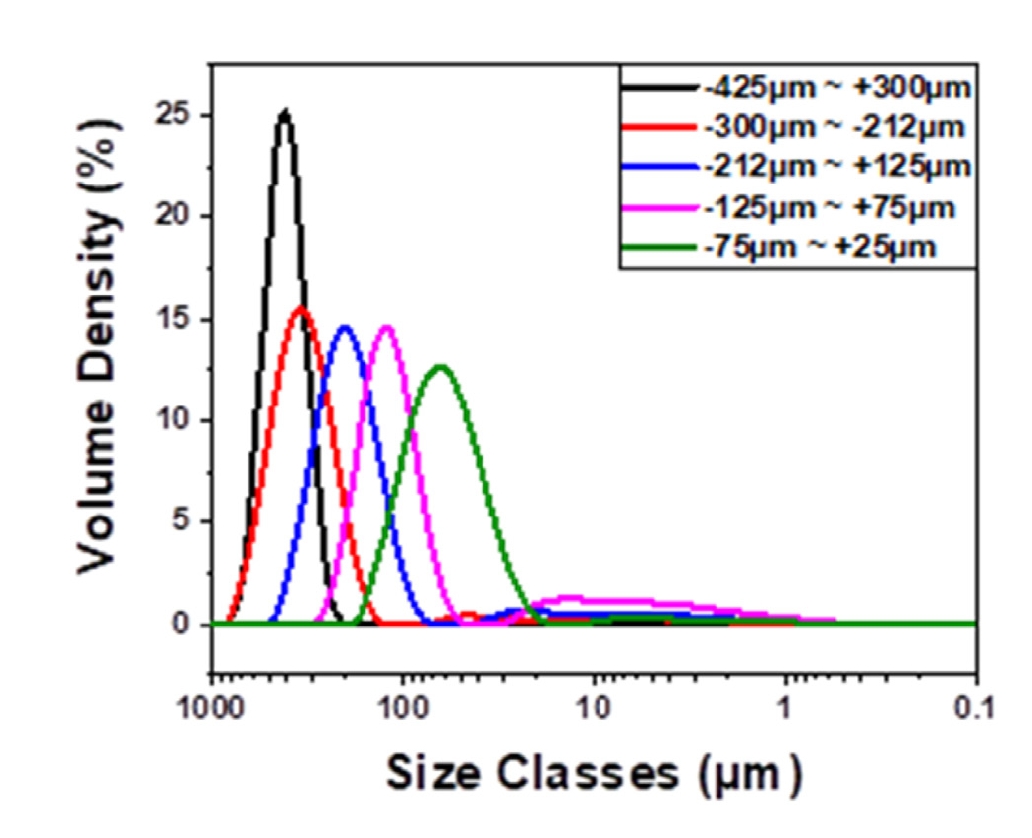
PDF - Carbonized blocks with different porosities were prepared by varying the particle size of the filler and subsequent impregnation. The impregnated carbonized blocks were re-carbonized. The use of smaller particles in the filler in the carbonized block was associated with larger porosity, smaller pore size, and a higher impregnation ratio. The block with the smallest average particle size (53 μm), CB-53, had a porosity of 35.9% and pores of approximately 40 μm, while the block with the largest average particle size (413 μm), CB-413, had a porosity of 30.5% and pores of approximately 150 μm. CB-53 had the highest bulk density, electrical resistivity, flexural strength, and impregnation ratio. This is due to the large porosity, which is believed to be due to the presence of more interfaces between particles during the re-carbonization of the impregnated carbonized block, resulting in a better pore-filling effect.
- [English]
- Design of Conductive Inks Containing Carbon Black and Silver Nanowires for Patternable Screen-Printing on Fabrics
- Seokhwan Kim, Geumseong Lee, Jinwoo Park, Dahye Shin, Ki-Il Park, Kyoung Jin Jung, Yuho Min
- J Powder Mater. 2024;31(6):500-507. Published online December 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00409
- 1,989 View
- 58 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
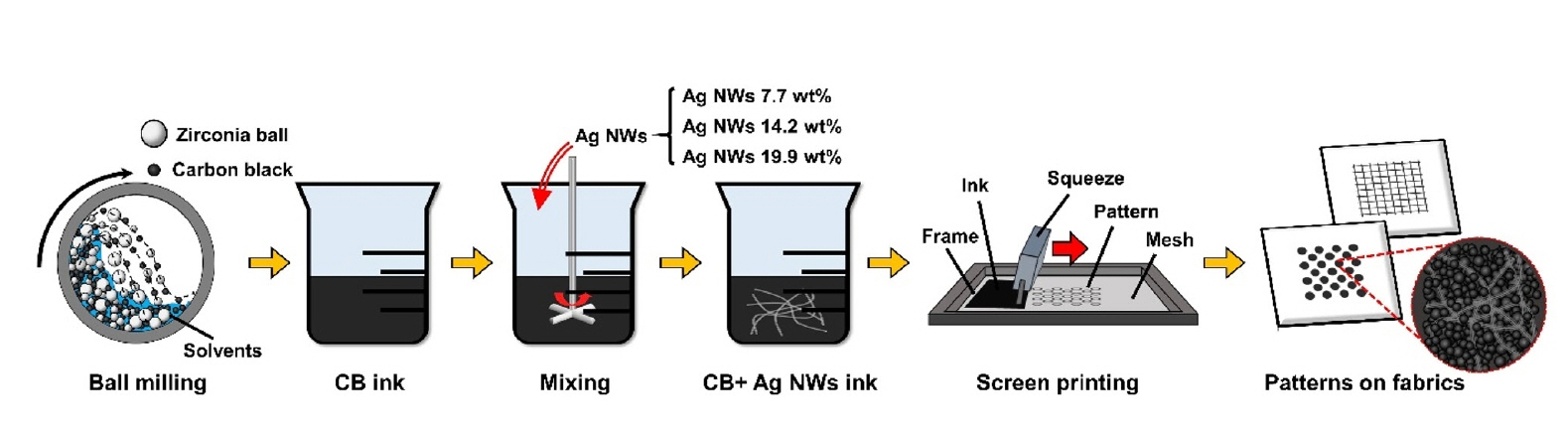
PDF - This study developed conductive inks composed of carbon black (CB) and silver nanowires (Ag NWs) for cost-effective screen-printing on fabrics. The Ag NW density within the CB matrix was precisely controlled, achieving tunable electrical conductivity with minimal Ag NW usage. The resulting inks were successfully patterned into shapes such as square grids and circles on textile surfaces, demonstrating excellent conductivity and fidelity. Adding 19.9 wt% Ag NWs reduced sheet resistance by ~92% compared to CB-only inks, highlighting the effectiveness and potential of this hybrid approach for cost-effective, high-performance textile-based electronics. The one-dimensional morphology of Ag NWs facilitated the formation of conductive percolation networks, creating efficient electron pathways within the CB matrix even at low loadings. This work advances the field of CB-based conductive inks and provides a scalable and practical method for producing functional, patterned electronic textiles.
-
Citations
Citations to this article as recorded by- Multifunctional Screen-Printed Conductive Inks: Design Principles, Performance Challenges, and Application Horizons
Nahid Islam, Manisha Das, Bashir Ahmed Johan, Syed Shaheen Shah, Atif Saeed Alzahrani, Md. Abdul Aziz
ACS Applied Electronic Materials.2025; 7(16): 7503. CrossRef
- Multifunctional Screen-Printed Conductive Inks: Design Principles, Performance Challenges, and Application Horizons
- [Korean]
- Synthesis of Carbon Coated Nickel Cobalt Sulfide Yolk-shell Microsphere and Their Application as Anode Materials for Sodium Ion Batteries
- Hyo Yeong Seo, Gi Dae Park
- J Powder Mater. 2023;30(5):387-393. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.387
- 789 View
- 15 Download
-
 Abstract
Abstract
 PDF
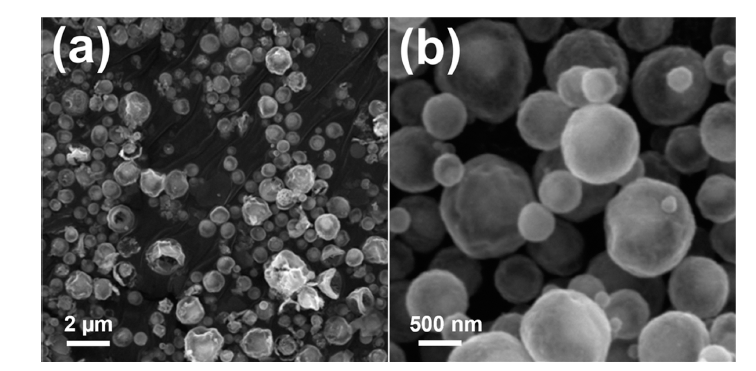
PDF Transition metal chalcogenides are promising cathode materials for next-generation battery systems, particularly sodium-ion batteries. Ni3Co6S8-pitch-derived carbon composite microspheres with a yolk-shell structure (Ni3Co6S8@C-YS) were synthesized through a three-step process: spray pyrolysis, pitch coating, and post-heat treatment process. Ni3Co6S8@C-YS exhibited an impressive reversible capacity of 525.2 mA h g-1 at a current density of 0.5 A g-1 over 50 cycles when employed as an anode material for sodium-ion batteries. However, Ni3Co6S8 yolk shell nanopowder (Ni3Co6S8-YS) without pitch-derived carbon demonstrated a continuous decrease in capacity during charging and discharging. The superior sodium-ion storage properties of Ni3Co6S8@C-YS were attributed to the pitchderived carbon, which effectively adjusted the size and distribution of nanocrystals. The carbon-coated yolk-shell microspheres proposed here hold potential for various metal chalcogenide compounds and can be applied to various fields, including the energy storage field.
- [Korean]
- Capacitance Enhancement and Evaluation of Gold-Deposited Carbon Nanotube Film Ion-Selective Electrode
- Do Youn Kim, Hanbyeol Son, Hyo-Ryoung Lim
- J Powder Mater. 2023;30(4):310-317. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.310
- 964 View
- 7 Download
-
 Abstract
Abstract
 PDF
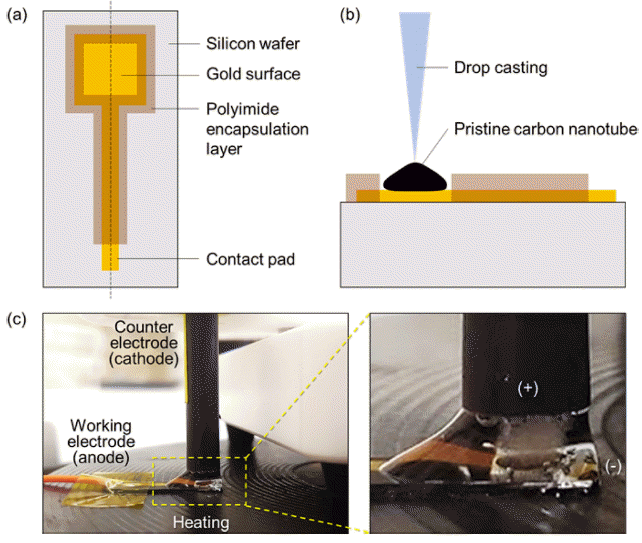
PDF Small-film-type ion sensors are garnering considerable interest in the fields of wearable healthcare and home-based monitoring systems. The performance of these sensors primarily relies on electrode capacitance, often employing nanocomposite materials composed of nano- and sub-micrometer particles. Traditional techniques for enhancing capacitance involve the creation of nanoparticles on film electrodes, which require cost-intensive and complex chemical synthesis processes, followed by additional coating optimization. In this study, we introduce a simple one-step electrochemical method for fabricating gold nanoparticles on a carbon nanotube (Au NP–CNT) electrode surface through cyclic voltammetry deposition. Furthermore, we assess the improvement in capacitance by distinguishing between the electrical double-layer capacitance and diffusion-controlled capacitance, thereby clarifying the principles underpinning the material design. The Au NP–CNT electrode maintains its stability and sensitivity for up to 50 d, signifying its potential for advanced ion sensing. Additionally, integration with a mobile wireless data system highlights the versatility of the sensor for health applications.
- [Korean]
- Research on the Manufacturing Technology for a PDMS Structure-Based Transpiration Generator Using Biomimetic Capillary Phenomenon
- Seung-Hwan Lee, Jeungjai Yun, So Hyun Baek, Yongbum Kwon, Yoseb Song, Bum Sung Kim, Yong-Ho Choa, Da-Woon Jeong
- J Powder Mater. 2023;30(3):268-275. Published online June 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.3.268
- 1,168 View
- 4 Download
-
 Abstract
Abstract
 PDF
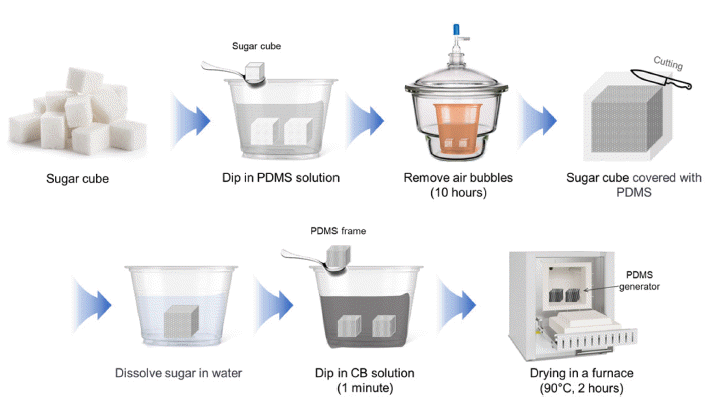
PDF The demand for energy is steadily rising because of rapid population growth and improvements in living standards. Consequently, extensive research is being conducted worldwide to enhance the energy supply. Transpiration power generation technology utilizes the vast availability of water, which encompasses more than 70% of the Earth's surface, offering the unique advantage of minimal temporal and spatial constraints over other forms of power generation. Various principles are involved in water-based energy harvesting. In this study, we focused on explaining the generation of energy through the streaming potential within the generator component. The generator was fabricated using sugar cubes, PDMS, carbon black, CTAB, and DI water. In addition, a straightforward and rapid manufacturing method for the generator was proposed. The PDMS generator developed in this study exhibits high performance with a voltage of 29.6 mV and a current of 8.29 μA and can generate power for over 40h. This study contributes to the future development of generators that can achieve high performance and long-term power generation.
- [Korean]
- Changes in Mechanical and Electrical Properties as a Function of Unidirectional Pressure Changes in Preforming While Isostatic Pressing for Graphite Block Fabrication
- Tae-Sub Byun, Dong-Pyo Jeon, Sang-Hye Lee, Sang-Woo Lee, Jae-Seung Roh
- J Powder Mater. 2023;30(1):35-40. Published online February 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.1.35
- 1,611 View
- 9 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
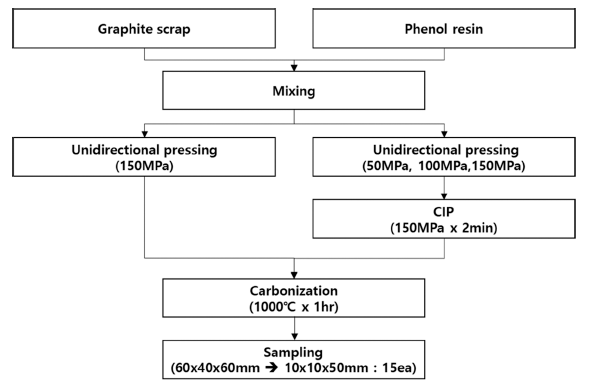
PDF In this study, a graphite block is fabricated using artificial graphite processing byproduct and phenolic resin as raw materials. Mechanical and electrical property changes are confirmed due to the preforming method. After fabricating preforms at 50, 100, and 150 MPa, CIP molding at 150 MPa is followed by heat treatment to prepare a graphite block. 150UP-CIP shows a 12.9% reduction in porosity compared with the 150 MPa preform. As the porosity is decreased, the bulk density, flexural strength, and shore hardness are increased by 14.9%, 102.4%, and 13.7%, respectively; and the deviation of density and electrical resistivity are decreased by 51.9% and 34.1%, respectively. Therefore, as the preforming pressure increases, the porosity decreases, and the electrical and mechanical properties improve.
-
Citations
Citations to this article as recorded by- Effect of Microstructural Change under Pressure during Isostatic Pressing on Mechanical and Electrical Properties of Isotropic Carbon Blocks
Tae-Sub Byun, Sang-Hye Lee, Suk-Hwan Kim, Jae-Seung Roh
Materials.2024; 17(2): 387. CrossRef - Effect of Pressure and Holding Time during Compression Molding on Mechanical Properties and Microstructure of Coke-Pitch Carbon Blocks
Sun-Ung Gwon, Sang-Hye Lee, U-Sang Youn, Jae-Seung Roh
Applied Sciences.2024; 14(2): 772. CrossRef
- Effect of Microstructural Change under Pressure during Isostatic Pressing on Mechanical and Electrical Properties of Isotropic Carbon Blocks
- [Korean]
- Developing Continuous Stabilization Process for Textile-Grade PAN Fiber-Based Carbon Fiber Using UV Irradiation
- Joon Ha Moon, Honggyu Seong, Jiseon Yoo, Se Youn Cho, Jaewon Choi
- J Powder Mater. 2022;29(5):418-423. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.418
- 921 View
- 5 Download
-
 Abstract
Abstract
 PDF
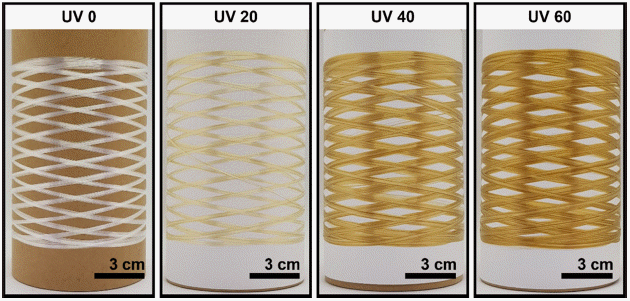
PDF Carbon fibers (CFs) are considered promising composite materials for various applications. However, the high cost of CFs (as much as $26 per kg) limits their practical use in the automobile and energy industries. In this study, we developed a continuous stabilization process for manufacturing low-cost CFs. We employed a textile-grade polyacrylonitrile (PAN) fiber as a low-cost precursor and UV irradiation technique to shorten the thermal stabilization time. We confirmed that UV irradiation on the textile-grade PAN fibers could lower the initial thermal stabilization temperature and also lead to a higher reaction. These resulted in a shorter overall stabilization time and enhancement of the tensile properties of textilegrade PAN-based CFs. Our study found that only 70 min of stabilization time with UV irradiation was required to prepare textile-grade PAN-based low-cost CFs with a tensile strength of 2.37 ± 0.22GPa and tensile modulus of 249 ± 5 GPa.
- [Korean]
- Comparison Study of Compact Titanium Oxide (c-TiO2) Powder Electron Transport Layer Fabrication for Carbon Electrode-based Perovskite Solar Cells
- Chae Young Woo, Hyung Woo Lee
- J Powder Mater. 2022;29(4):297-302. Published online August 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.4.297
- 820 View
- 7 Download
-
 Abstract
Abstract
 PDF
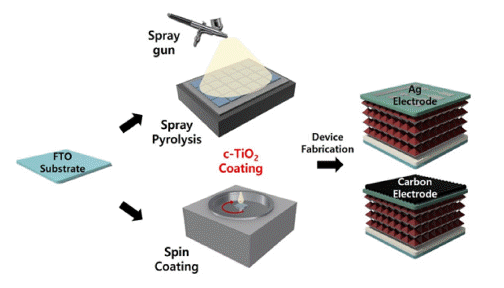
PDF This study compares the characteristics of a compact TiO2 (c-TiO2) powdery film, which is used as the electron transport layer (ETL) of perovskite solar cells, based on the manufacturing method. Additionally, its efficiency is measured by applying it to a carbon electrode solar cell. Spin-coating and spray methods are compared, and spraybased c-TiO2 exhibits superior optical properties. Furthermore, surface analysis by scanning electron microscopy (SEM) and atomic force microscopy (AFM) exhibits the excellent surface properties of spray-based TiO2. The photoelectric conversion efficiency (PCE) is 14.31% when applied to planar perovskite solar cells based on metal electrodes. Finally, carbon nanotube (CNT) film electrode-based solar cells exhibits a 76% PCE compared with that of metal electrodebased solar cells, providing the possibility of commercialization.
- [Korean]
- Spark Plasma Sintering Method to Replace Carburizing Process
- Junhyub Jeon, Junho Lee, Namhyuk Seo, Seung Bae Son, Jae-Gil Jung, Seok-Jae Lee
- J Powder Mater. 2022;29(3):219-225. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.219
- 529 View
- 4 Download
-
 Abstract
Abstract
 PDF
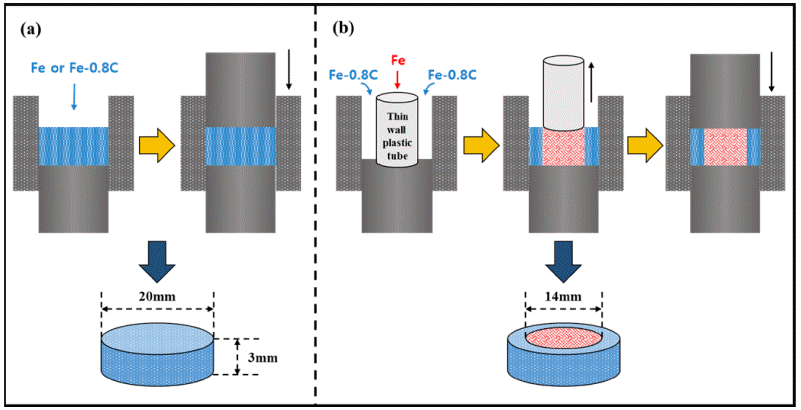
PDF An alternative fabrication method for carburizing steel using spark plasma sintering (SPS) is investigated. The sintered carburized sample, which exhibits surface modification effects such as carburizing, sintered Fe, and sintered Fe–0.8 wt.%C alloys, is fabricated using SPS. X-ray diffraction and micro Vickers tests are employed to confirm the phase and properties. Finite element analysis is performed to evaluate the change in hardness and analyze the carbon content and residual stress of the carburized sample. The change in the hardness of the carburized sample has the same tendency to predict hardness. The difference in hardness between the carburized sample and the predicted value is also discussed. The carburized sample exhibits a compressive residual stress at the surface. These results indicate that the carburized sample experiences a surface modification effect without carburization. Field emission scanning electron microscopy is employed to verify the change in phase. A novel fabrication method for altering the carburization is successfully proposed. We expect this fabrication method to solve the problems associated with carburization.
- [Korean]
- Preparation of CoFe2O4 Nanoparticle Decorated on Electrospun Carbon Nanofiber Composite Electrodes for Supercapacitors
- Hyewon Hwang, Seoyeon Yuk, Minsik Jung, Dongju Lee
- J Korean Powder Metall Inst. 2021;28(6):470-477. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.470
- 749 View
- 4 Download
-
 Abstract
Abstract
 PDF
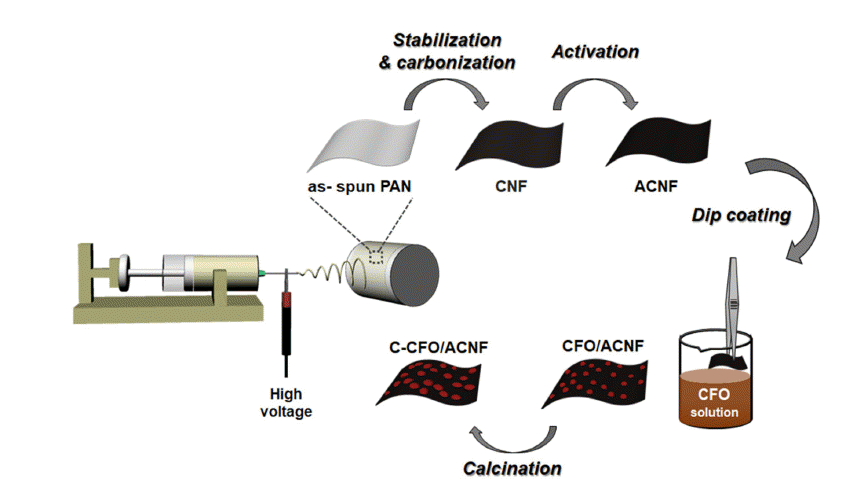
PDF Energy storage systems should address issues such as power fluctuations and rapid charge-discharge; to meet this requirement, CoFe2O4 (CFO) spinel nanoparticles with a suitable electrical conductivity and various redox states are synthesized and used as electrode materials for supercapacitors. In particular, CFO electrodes combined with carbon nanofibers (CNFs) can provide long-term cycling stability by fabricating binder-free three-dimensional electrodes. In this study, CFO-decorated CNFs are prepared by electrospinning and a low-cost hydrothermal method. The effects of heat treatment, such as the activation of CNFs (ACNFs) and calcination of CFO-decorated CNFs (C-CFO/ACNFs), are investigated. The C-CFO/ACNF electrode exhibits a high specific capacitance of 142.9 F/g at a scan rate of 5 mV/s and superior rate capability of 77.6% capacitance retention at a high scan rate of 500 mV/s. This electrode also achieves the lowest charge transfer resistance of 0.0063 Ω and excellent cycling stability (93.5% retention after 5,000 cycles) because of the improved ion conductivity by pathway formation and structural stability. The results of our work are expected to open a new route for manufacturing hybrid capacitor electrodes containing the C-CFO/ACNF electrode that can be easily prepared with a low-cost and simple process with enhanced electrochemical performance.
- [Korean]
- Development of Aluminum Matrix Composites Containing Nano-carbon Materials
- Jungjoon Kim, Daeyoung Kim, Hyunjoo Choi
- J Korean Powder Metall Inst. 2021;28(3):253-258. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.253
- 860 View
- 6 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
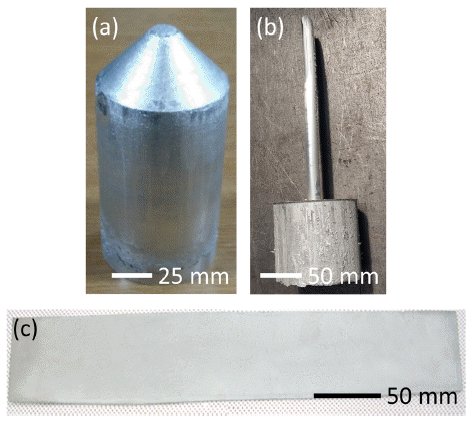
PDF There is increasing demand for the development of a new material with high strength, high stiffness, and good electrical conductivity that can be used for high-voltage direct current cables. In this study, we develop aluminumbased composites containing C60 fullerenes, carbon nanotubes, or graphene using a powder metallurgical route and evaluate their strength, stiffness, coefficient of thermal expansion, and electrical conductivity. By optimizing the process conditions, a material with a tensile strength of 800 MPa, an elastic modulus of 90 GPa, and an electrical conductivity of 40% IACS is obtained, which may replace iron-core cables. Furthermore, by designing the type and volume fraction of the reinforcement, a material with a tensile strength of 380 MPa, elastic modulus of 80 GPa, and electrical conductivity of 54% IACS is obtained, which may compete with AA 6201 aluminum alloys for use in all-aluminum conductor cables.
-
Citations
Citations to this article as recorded by- Synergistic strengthening of aluminum with SiC by grain refinement and dispersion hardening
Kanhu C. Nayak, Juyeon Han, Suwon Park, Miran Joo, Kon‐Bae Lee, Donghyun Bae, Hyunjoo Choi
Journal of the American Ceramic Society.2023; 106(12): 7340. CrossRef - Synergetic effect of milling speed and duration on particle morphology and mechanical properties of nanocrystalline Al matrix containing SiC
K.C. Nayak, J.Y. Han, C.H. Jung, M.R. Joo, K.B. Lee, D.H. Bae, H.J. Choi
Powder Metallurgy.2023; 66(5): 519. CrossRef
- Synergistic strengthening of aluminum with SiC by grain refinement and dispersion hardening
- [Korean]
- RBSC Prepared by Si Melt Infiltration into the Y2O3 Added Carbon Preform
- Min-Ho Jang, Kyeong-Sik Cho
- J Korean Powder Metall Inst. 2021;28(1):51-58. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.51
- 1,443 View
- 15 Download
-
 Abstract
Abstract
 PDF
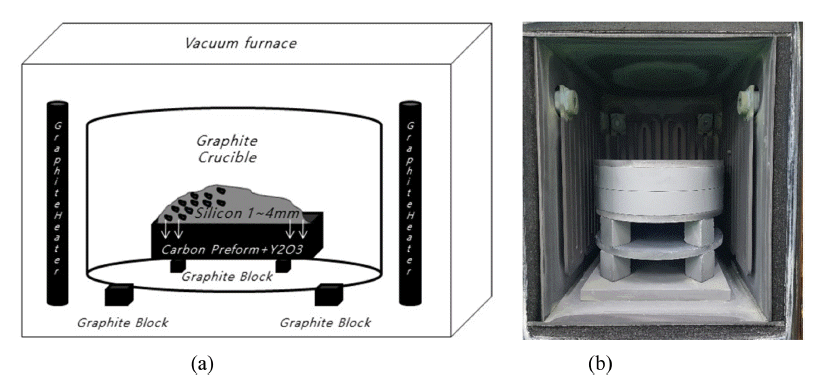
PDF The conversion of carbon preforms to dense SiC by liquid infiltration is a prospectively low-cost and reliable method of forming SiC-Si composites with complex shapes and high densities. Si powder was coated on top of a 2.0wt .% Y2O3-added carbon preform, and reaction bonded silicon carbide (RBSC) was prepared by infiltrating molten Si at 1,450°C for 1-8 h. Reactive sintering of the Y2O3-free carbon preform caused Si to be pushed to one side, thereby forming cracking defects. However, when prepared from the Y2O3-added carbon preform, a SiC-Si composite in which Si is homogeneously distributed in the SiC matrix without cracking can be produced. Using the Si + C → SiC reaction at 1,450°C, 3C and 6H SiC phases, crystalline Si, and Y2O3 were generated based on XRD analysis, without the appearance of graphite. The RBSC prepared from the Y2O3-added carbon preform was densified by increasing the density and decreasing the porosity as the holding time increased at 1,450°C. Dense RBSC, which was reaction sintered at 1,450°C for 4 h from the 2.0wt.% Y2O3-added carbon preform, had an apparent porosity of 0.11% and a relative density of 96.8%.
- [Korean]
- Fabrication of Macro-porous Carbon Foams from Spherical Phenolic Resin Powder and Furfuryl Alcohol by Casting Molding
- Hyeondeok Jeong, Seiki Kim
- J Korean Powder Metall Inst. 2019;26(6):502-507. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.502
- 547 View
- 1 Download
-
 Abstract
Abstract
 PDF
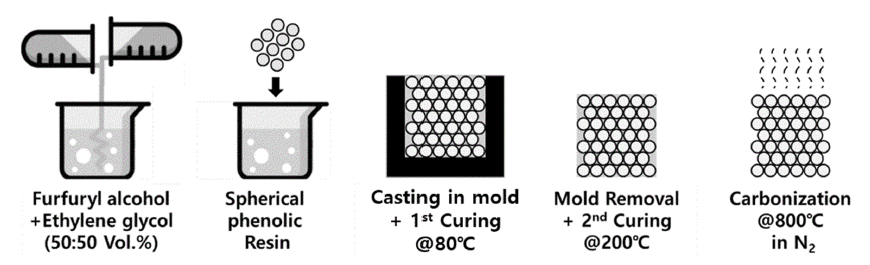
PDF Macro-porous carbon foams are fabricated using cured spherical phenolic resin particles as a matrix and furfuryl alcohol as a binder through a simple casting molding. Different sizes of the phenolic resin particles from 100–450 μm are used to control the pore size and structure. Ethylene glycol is additionally added as a pore-forming agent and oxalic acid is used as an initiator for polymerization of furfuryl alcohol. The polymerization is performed in two steps; at 80°C and 200°C in an ambient atmosphere. The carbonization of the cured body is performed under Nitrogen gas flow (0.8 L/min) at 800°C for 1 h. Shrinkage rate and residual carbon content are measured by size and weight change after carbonization. The pore structures are observed by both electron and optical microscope and compared with the porosity results achieved by the Archimedes method. The porosity is similar regardless of the size of the phenolic resin particles. On the other hand, the pore size increases in proportion to the phenol resin size, which indicates that the pore structure can be controlled by changing the raw material particle size.
- [English]
- A Separator with Activated Carbon Powder Layer to Enhance the Performance of Lithium-Sulfur Batteries
- Duc-Luong Vu, Jae-Won Lee
- J Korean Powder Metall Inst. 2018;25(6):466-474. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.466
- 1,124 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
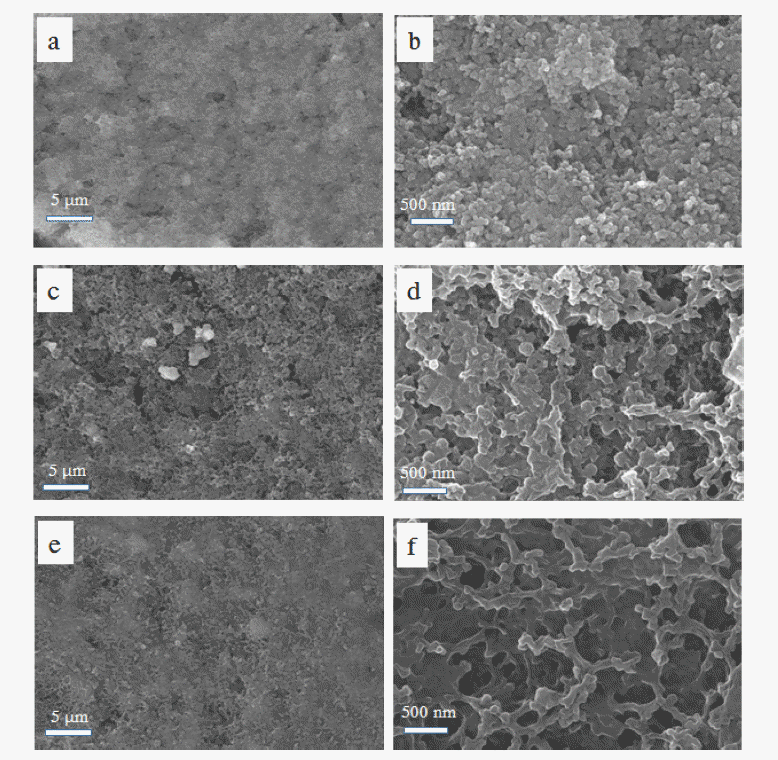
PDF The high theoretical energy density (2600 Wh kg−1) of Lithium-sulfur batteries and the high theoretical capacity of elemental sulfur (1672 mAh g−1) attract significant research attention. However, the poor electrical conductivity of sulfur and the polysulfide shuttle effect are chronic problems resulting in low sulfur utilization and poor cycling stability. In this study, we address these problems by coating a polyethylene separator with a layer of activated carbon powder. A lithium-sulfur cell containing the activated carbon powder-coated separator exhibits an initial specific discharge capacity of 1400 mAh g−1 at 0.1 C, and retains 63% of the initial capacity after 100 cycles at 0.2 C, whereas the equivalent cell with a bare separator exhibits a 1200 mAh g−1 initial specific discharge capacity, and 50% capacity retention under the same conditions. The activated carbon powder-coated separator also enhances the rate capability. These results indicate that the microstructure of the activated carbon powder layer provides space for the sulfur redox reaction and facilitates fast electron transport. Concurrently, the activated carbon powder layer traps and reutilizes any polysulfides dissolved in the electrolyte. The approach presented here provides insights for overcoming the problems associated with lithium-sulfur batteries and promoting their practical use.
-
Citations
Citations to this article as recorded by- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
Yueting Zhu, Jingjing Wang, Yanshu Wang, Ying Zhu, Yixuan Li, Shicheng Zhao
Ionics.2022; 28(4): 1693. CrossRef - High thermal stability multilayered electrolyte complexes via layer-by-layer for long-life lithium-sulfur battery
Jing Wang, Yufan Li, Xianmei Deng, Lei Yan, Zhiqiang Shi
Ionics.2020; 26(11): 5481. CrossRef
- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
- [Korean]
- Effects of Morphologies of Carbon Nanomaterials on Conductivity of Composites Containing Copper/Carbon Nanomaterial Hybrid Fillers
- Yeonjoo Lee, Sung-uk Hong, Hyunjoo Choi
- J Korean Powder Metall Inst. 2018;25(5):435-440. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.435
- 555 View
- 1 Download
-
 Abstract
Abstract
 PDF
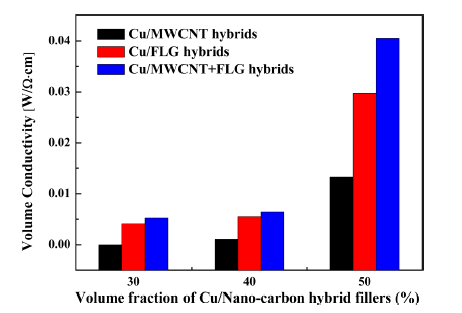
PDF In the present study, we develop a conductive copper/carbon nanomaterial additive and investigate the effects of the morphologies of the carbon nanomaterials on the conductivities of composites containing the additive. The conductive additive is prepared by mechanically milling copper powder with carbon nanomaterials, namely, multi-walled carbon nanotubes (MWCNTs) and/or few-layer graphene (FLG). During the milling process, the carbon nanomaterials are partially embedded in the surfaces of the copper powder, such that electrically conductive pathways are formed when the powder is used in an epoxy-based composite. The conductivities of the composites increase with the volume of the carbon nanomaterial. For a constant volume of carbon nanomaterial, the FLG is observed to provide more conducting pathways than the MWCNTs, although the optimum conductivity is obtained when a mixture of FLG and MWCNTs is used.
- [English]
- A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
- Jei-Pil Wang, Jae-Jung Pyo, Se-Ho Ahn, Dong-Hyeon Choi, Byeong-Woo Lee, Dong-Won Lee
- J Korean Powder Metall Inst. 2018;25(4):296-301. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.296
- 3,483 View
- 76 Download
- 11 Citations
-
 Abstract
Abstract
 PDF
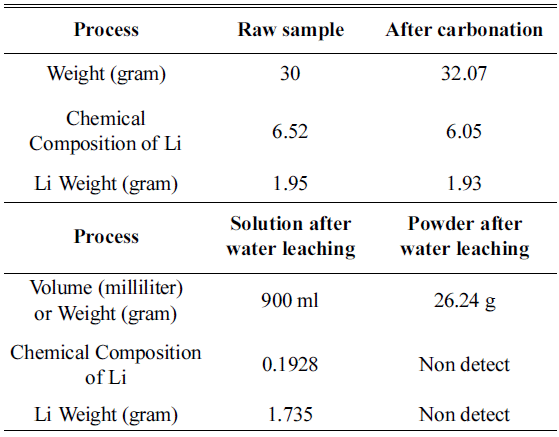
PDF In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered.
-
Citations
Citations to this article as recorded by- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
Amir Hossein Mohammad Zadeh, Spencer Cunnigham, Devon Gray, Sevan Bedrossian, Baian Almusned, Jeffrey Daniel Henderson, Gisele Azimi
Waste Management.2026; 212: 115339. CrossRef - Hydrogen Reduction of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries
Jae-Ho Hwang, Sang-Yeop Lee, So-Yeong Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(3): 34. CrossRef - Reduction Roasting of Cathode Materials of NCM Based Lithium-ion Batteries Using CH4(g)
Jae-Ho Hwang, Sang-Yeop Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(4): 48. CrossRef - High‐Rate Rechargeable Li/SOCl2 Batteries Enabled by Cobalt Phthalocyanine Cathodic Catalysts
Yingxuan Song, Haibo Ouyang, Zhanwei Xu, Zeyang Zhang, Kang Li, Jianfeng Huang, Zhi Li, Tian Wang, Jun Yang
Small.2025;[Epub] CrossRef - Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef - Metals Recovery from Spent Lithium-ion Batteries Cathode Via Hydrogen Reduction-water Leaching-carbothermic or Hydrogen Reduction Process
Tahereh Rostami, Behnam Khoshandam
Mining, Metallurgy & Exploration.2024; 41(3): 1485. CrossRef - Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(5): 923. CrossRef - Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(12): 2000. CrossRef - Environmentally Friendly Recovery of Lithium from Lithium–Sulfur Batteries
Lilian Schwich, Bernd Friedrich
Metals.2022; 12(7): 1108. CrossRef - Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Lilian Schwich, Tom Schubert, Bernd Friedrich
Metals.2021; 11(2): 177. CrossRef - Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem
Jiadong Yu, Quanyin Tan, Jinhui Li
Journal of Cleaner Production.2020; 255: 120269. CrossRef
- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
- [English]
- Development of Carbon Nanotube-copper Hybrid Powder as Conductive Additive
- Minjae Lee, Seoungjun Haa, Yeonjoo Lee, Haneul Jang, Hyunjoo Choi
- J Korean Powder Metall Inst. 2018;25(4):291-295. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.291

- 1,366 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF A conductive additive is prepared by dispersing multi-walled carbon nanotubes (MWCNTs) on Cu powder by mechanical milling and is distributed in epoxy to enhance its electrical conductivity. During milling, the MWCNTs are dispersed and partially embedded on the surface of the Cu powder to provide electrically conductive pathways within the epoxy-based composite. The degree of dispersion of the MWCNTs is controlled by varying the milling medium and the milling time. The MWCNTs are found to be more homogeneously dispersed when solvents (particularly, non-polar solvent, i.e., NMP) are used. MWCNTs gradually disperse on the surface of Cu powder because of the plastic deformation of the ductile Cu powder. However, long-time milling is found to destroy the molecular structure of MWCNTs, instead of effectively dispersing the MWCNTs more uniformly. Thus, the epoxy composite film fabricated in this study exhibits a higher electrical conductivity than 1.1 S/cm.
-
Citations
Citations to this article as recorded by- Effects of Morphologies of Carbon Nanomaterials on Conductivity of Composites Containing Copper/Carbon Nanomaterial Hybrid Fillers
Yeonjoo Lee, Sung-uk Hong, Hyunjoo Choi
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 435. CrossRef
- Effects of Morphologies of Carbon Nanomaterials on Conductivity of Composites Containing Copper/Carbon Nanomaterial Hybrid Fillers
- [English]
- Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
- Bong Gill Choi, MinHo Yang
- J Korean Powder Metall Inst. 2018;25(3):203-207. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2017.25.3.203
- 627 View
- 3 Download
-
 Abstract
Abstract
 PDF
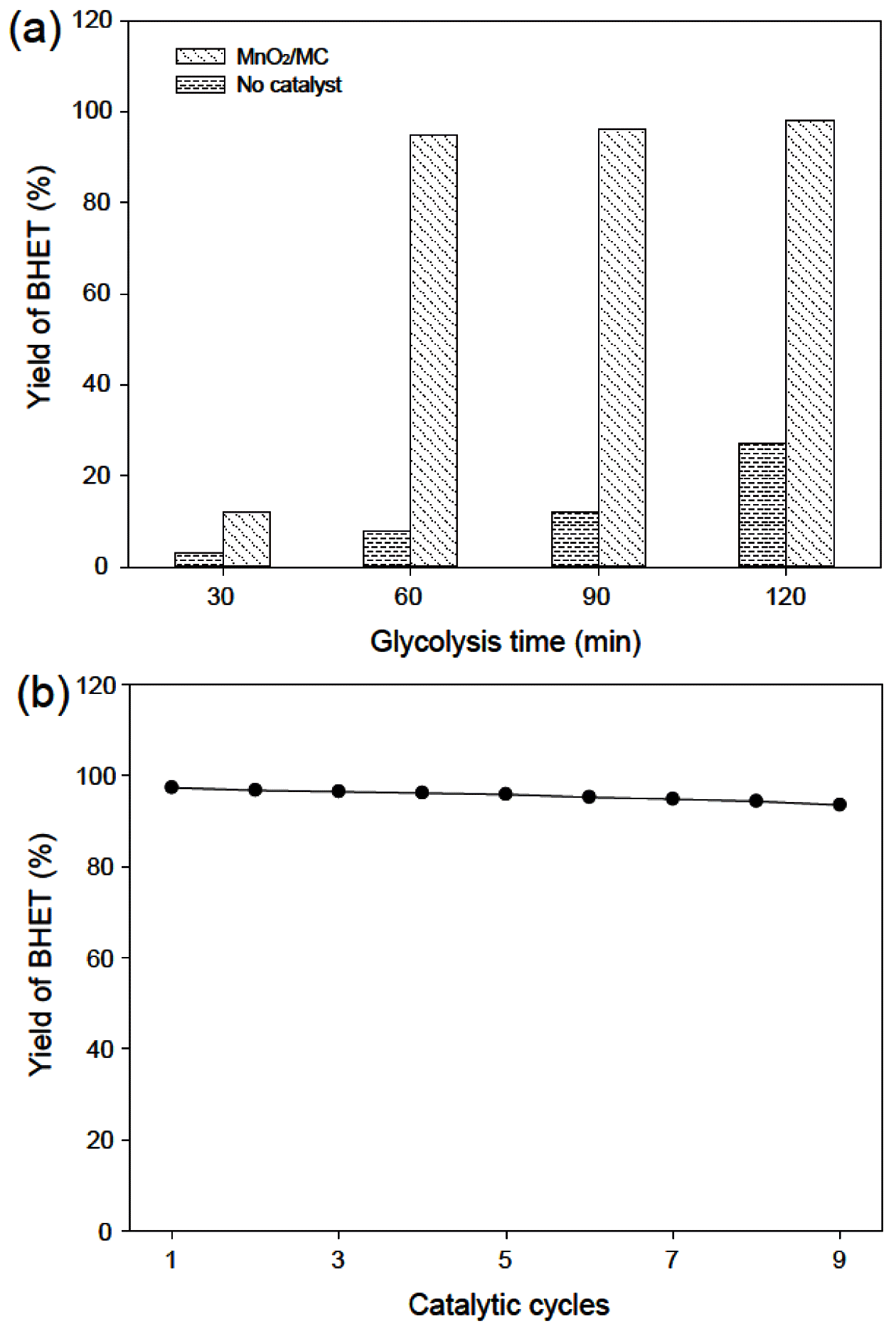
PDF Plastic pollution is threatening human health and ecosystems, resulting in one of the biggest challenges that humanity has ever faced. Therefore, this study focuses on the preparation of macroporous carbon from biowaste (MC)-supported manganese oxide (MnO2) as an efficient, reusable, and robust catalyst for the recycling of poly(ethylene terephthalate) (PET) waste. As-prepared MnO2/MC composites have a hierarchical pore network and a large surface area (376.16 m2/g) with a narrow size distribution. MnO2/MC shows a maximum yield (98%) of bis(2-hydroxyethyl)terephthalate (BHET) after glycolysis reaction for 120 min. Furthermore, MnO2/MC can be reused at least nine times with a negligible decrease in BHET yield. Based on this remarkable catalytic performance, we expect that MnO2-based heterogeneous catalysts have the potential to be introduced into the PET recycling industry.
- [Korean]
- Fabrication of CNT dispersed Cu matrix composites by wet mixing and spark plasma sintering process
- Seungchan Cho, Ilguk Jo, Sang-Bok Lee, Sang-Kwan Lee, Moonhee Choi, Jehong Park, Hansang Kwon, Yangdo Kim
- J Korean Powder Metall Inst. 2018;25(2):158-164. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.158

- 967 View
- 18 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Multi-walled carbon nanotube (MWCNT)–copper (Cu) composites are successfully fabricated by a combination of a binder-free wet mixing and spark plasma sintering (SPS) process. The SPS is performed under various conditions to investigate optimized processing conditions for minimizing the structural defects of CNTs and densifying the MWCNT–Cu composites. The electrical conductivities of MWCNT–Cu composites are slightly increased for compositions containing up to 1 vol.% CNT and remain above the value for sintered Cu up to 2 vol.% CNT. Uniformly dispersed CNTs in the Cu matrix with clean interfaces between the treated MWCNT and Cu leading to effective electrical transfer from the treated MWCNT to the Cu is believed to be the origin of the improved electrical conductivity of the treated MWCNT–Cu composites. The results indicate the possibility of exploiting CNTs as a contributing reinforcement phase for improving the electrical conductivity and mechanical properties in the Cu matrix composites.
-
Citations
Citations to this article as recorded by- Proposing Machine Learning Models Suitable for Predicting Open Data Utilization
Junyoung Jeong, Keuntae Cho
Sustainability.2024; 16(14): 5880. CrossRef
- Proposing Machine Learning Models Suitable for Predicting Open Data Utilization
- [Korean]
- The Microstructure and Mechanical Properties of Y2O3-Dispersed Fe-C and Fe-CNT Sintered Steels
- Jin Young Lim, Jung-Ho Ahn
- J Korean Powder Metall Inst. 2017;24(4):298-301. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.298
- 626 View
- 1 Download
-
 Abstract
Abstract
 PDF
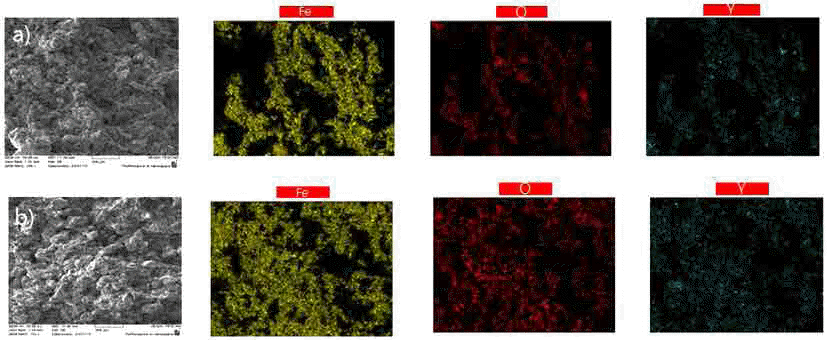
PDF In the present work, we use multiwall carbon nanotubes (MWCNT) as the starting material for the fabrication of sintered carbon steel. A comparison is made with conventionally sintered carbon steel, where graphite is used as the starting material. Milling is performed using a horizontal mill sintered in a vacuum furnace. We analyze the grain size, number of pores, X-ray diffraction patterns, and microstructure. Changes in the physical properties are determined by using the Archimedes method and Vickers hardness measurements. The result shows that the use of MWCNTs instead of graphite significantly reduces the size and volume of the pores as well as the grain size after sintering. The addition of Y2O3.to the Fe-MWCNT samples further inhibits the growth of grains.
- [English]
- Expansion of Multi-wall Carbon Nanotubes and its Lithium Storage Property
- Jung-Ho Ahn, Jeong-Seok Ahn
- J Korean Powder Metall Inst. 2017;24(4):275-278. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.275
- 809 View
- 2 Download
-
 Abstract
Abstract
 PDF
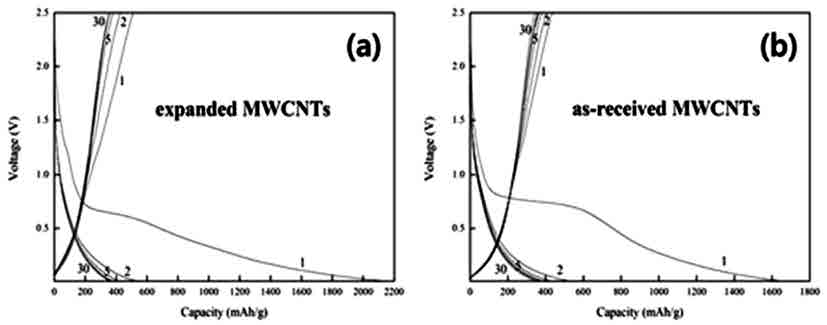
PDF In the present work, we apply a technique that has been used for the expansion of graphite to multiwall carbon nanotubes (MWCNT). The nanotubes are rapidly heated for a short duration, followed by immersion in acid solution, so that they undergo expansion. The diameter of the expanded CNTs is 5-10 times larger than that of the asreceived nanotubes. This results in considerable swelling of the CNTs and opening of the tube tips, which may facilitate the accessibility of lithium ions into the inner holes and the interstices between the nanotube walls. The Li-ion storage capacity of the expanded nanotubes is measured by using the material as an anode in Li-ion cells. The result show that the discharge capacity of the expanded nanotubes in the first cycle is as high as 2,160 mAh/g, which is about 28% higher than that of the un-treated MWCNT anode. However, the charge/discharge capacity quickly drops in subsequent cycles and finally reaches equilibrium values of ~370 mAh/g. This is possibly due to the destruction of the lattice structures by repeated intercalation of Li ions.
- [Korean]
- Synthesis of Nitrogen-doped Carbon Nanofibers for Oxygen Reduction Reaction
- Geon-Hyoung An, Eun-Hwan Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(6):420-425. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.420

- 647 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF N-doped carbon nanofibers as catalysts for oxygen-reduction reactions are synthesized using electrospinning and carbonization. Their morphologies, structures, chemical bonding states, and electrochemical performance are characterized. The optimized N-doped carbon nanofibers exhibit graphitization of carbon nanofibers and an increased nitrogen doping as well as a uniform network structure. In particular, the optimized N-doped carbon nanofibers show outstanding catalytic activity for oxygen-reduction reactions, such as a half-wave potential (E1/2) of 0.43 V, kinetic limiting current density of 6.2 mA cm-2, electron reduction pathways (n = 3.1), and excellent long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 13 mV. The improvement in the electrochemical performance results from the synergistic effect of the graphitization of carbon nanofibers and the increased amount of nitrogen doping.
- [Korean]
- Fabrication of Carbon-coated Tin Nano-powders by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ju-Suck Song, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2016;23(4):317-324. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.317
- 999 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
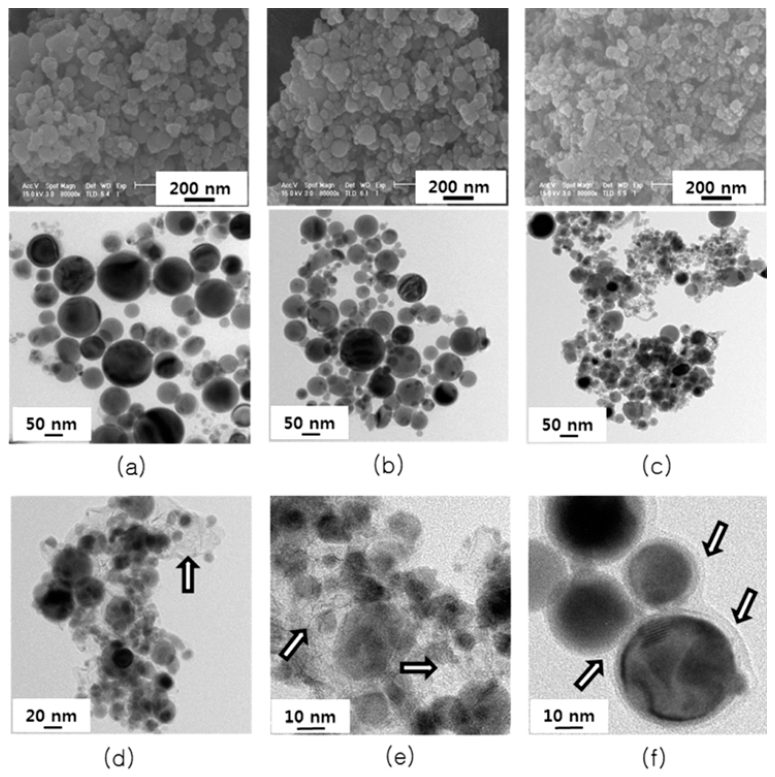
PDF Tin is one of the most promising anode materials for next-generation lithium-ion batteries with a high energy density. However, the commercialization of tin-based anodes is still hindered due to the large volume change (over 260%) upon lithiation/delithiation cycling. To solve the problem, many efforts have been focused on enhancing structural stability of tin particles in electrodes. In this work, we synthesize tin nano-powders with an amorphous carbon layer on the surface and surroundings of the powder by electrical wire explosion in alcohol-based liquid media at room temperature. The morphology and microstructures of the powders are characterized by scanning electron microscopy, Xray diffraction, Raman spectroscopy, and transmission electron microscopy. The electrochemical properties of the powder for use as an anode material for lithium-ion battery are evaluated by cyclic voltammetry and a galvanometric dischargecharge method. It is shown that the carbon-coated tin nano-powders prepared in hexanol media exhibit a high initial charge specific capacity of 902 mAh/g and a high capacity retention of 89% after 50 cycles.
-
Citations
Citations to this article as recorded by- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
Ji-Seub Choi, Yeon-Ju Lee, Hoi-Jin Lee, Gyu-Bong Cho, Jai-Won Byeon, Hyo-Jun Ahn, Ki-Won Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Journal of Alloys and Compounds.2020; 843: 155892. CrossRef - Fabrication of multilayer graphene-encapsulated Sn/SnO2 nanocomposite as an anode material for lithium-ion batteries and its electrochemical properties
Ju-Seok Song, Gyu-Bong Cho, Ki-Won Kim, Hyo-Jun Ahn, Hye-Sung Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Applied Surface Science.2019; 481: 736. CrossRef
- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
- [Korean]
- The Effect of Diffusion Barrier and thin Film Deposition Temperature on Change of Carbon Nanotubes Length
- Soon-kyu Hong, Hyung Woo Lee
- J Korean Powder Metall Inst. 2016;24(3):248-253. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2017.24.3.248

- 581 View
- 1 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, we investigate the effect of the diffusion barrier and substrate temperature on the length of carbon nanotubes. For synthesizing vertically aligned carbon nanotubes, thermal chemical vapor deposition is used and a substrate with a catalytic layer and a buffer layer is prepared using an e-beam evaporator. The length of the carbon nanotubes synthesized on the catalytic layer/diffusion barrier on the silicon substrate is longer than that without a diffusion barrier because the diffusion barrier prevents generation of silicon carbide from the diffusion of carbon atoms into the silicon substrate. The deposition temperature of the catalyst and alumina are varied from room temperature to 150°C, 200°C, and 250°C. On increasing the substrate temperature on depositing the buffer layer on the silicon substrate, shorter carbon nanotubes are obtained owing to the increased bonding force between the buffer layer and silicon substrate. The reason why different lengths of carbon nanotubes are obtained is that the higher bonding force between the buffer layer and the substrate layer prevents uniformity of catalytic islands for synthesizing carbon nanotubes.
-
Citations
Citations to this article as recorded by- A Study on Residual Powder Removing Technique of Multi-Layered Graphene Based on Graphene One-Step Transfer Process
Chae-young Woo, Yeongsu Jo, Soon-kyu Hong, Hyung Woo Lee
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 11. CrossRef - Fabrication of robust, ultrathin and light weight, hydrophilic, PVDF-CNT membrane composite for salt rejection
Vivek Dhand, Soon Kyu Hong, Luhe Li, Jong-Man Kim, Soo Hyung Kim, Kyong Yop Rhee, Hyung Woo Lee
Composites Part B: Engineering.2019; 160: 632. CrossRef
- A Study on Residual Powder Removing Technique of Multi-Layered Graphene Based on Graphene One-Step Transfer Process
- [Korean]
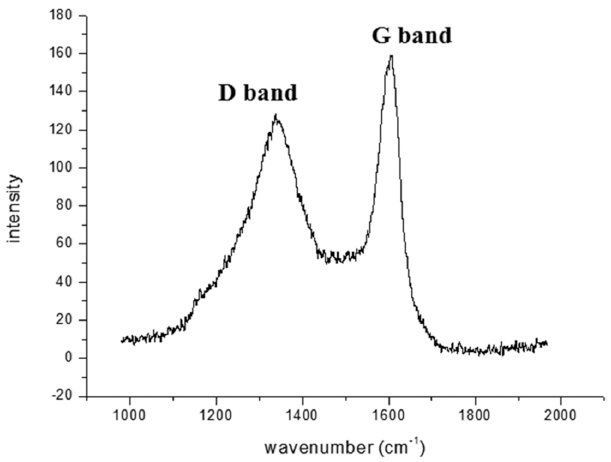
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2016;23(3):235-239. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.235
- 489 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF This study is performed to fabricate a Ti porous body by freeze drying process using titanium hydride (TiH2) powder and camphene. Then, the Ti porous body is employed to synthesize carbon nanotubes (CNTs) using thermal catalytic chemical vapor deposition (CCVD) with Fe catalyst and methane (CH4) gas to increase the specific surface area. The synthesized Ti porous body has 100 μm-sized macropores and 10-30 μm-sized micropores. The synthesized CNTs have random directions and are entangled with adjacent CNTs. The CNTs have a bamboo-like structure, and their average diameter is about 50 nm. The Fe nano-particles observed at the tip of the CNTs indicate that the tip growth model is applicable. The specific surface area of the CNT-coated Ti porous body is about 20 times larger than that of the raw Ti porous body. These CNT-coated Ti porous bodies are expected to be used as filters or catalyst supports.
- [Korean]
- Consolidation to Bulk Ceramic Bodies from Oyster Shell Powder
- Kyeong-Sik Cho, Hyun-Kwuon Lee, Jae Hong Min
- J Korean Powder Metall Inst. 2016;23(3):221-227. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.221
- 1,515 View
- 10 Download
-
 Abstract
Abstract
 PDF
PDF Waste oyster shells create several serious problems; however, only some parts of them are being utilized currently. The ideal solution would be to convert the waste shells into a product that is both environmentally beneficial and economically viable. An experimental study is carried out to investigate the recycling possibilities for oyster shell waste. Bulk ceramic bodies are produced from the oyster shell powder in three sequential processes. First, the shell powder is calcined to form calcium oxide CaO, which is then slaked by a slaking reaction with water to produce calcium hydroxide Ca(OH)2. Then, calcium hydroxide powder is formed by uniaxial pressing. Finally, the calcium hydroxide compact is reconverted to calcium carbonate via a carbonation reaction with carbon dioxide released from the shell powder bed during firing at 550°C. The bulk body obtained from waste oyster shells could be utilized as a marine structural porous material.
- [English]
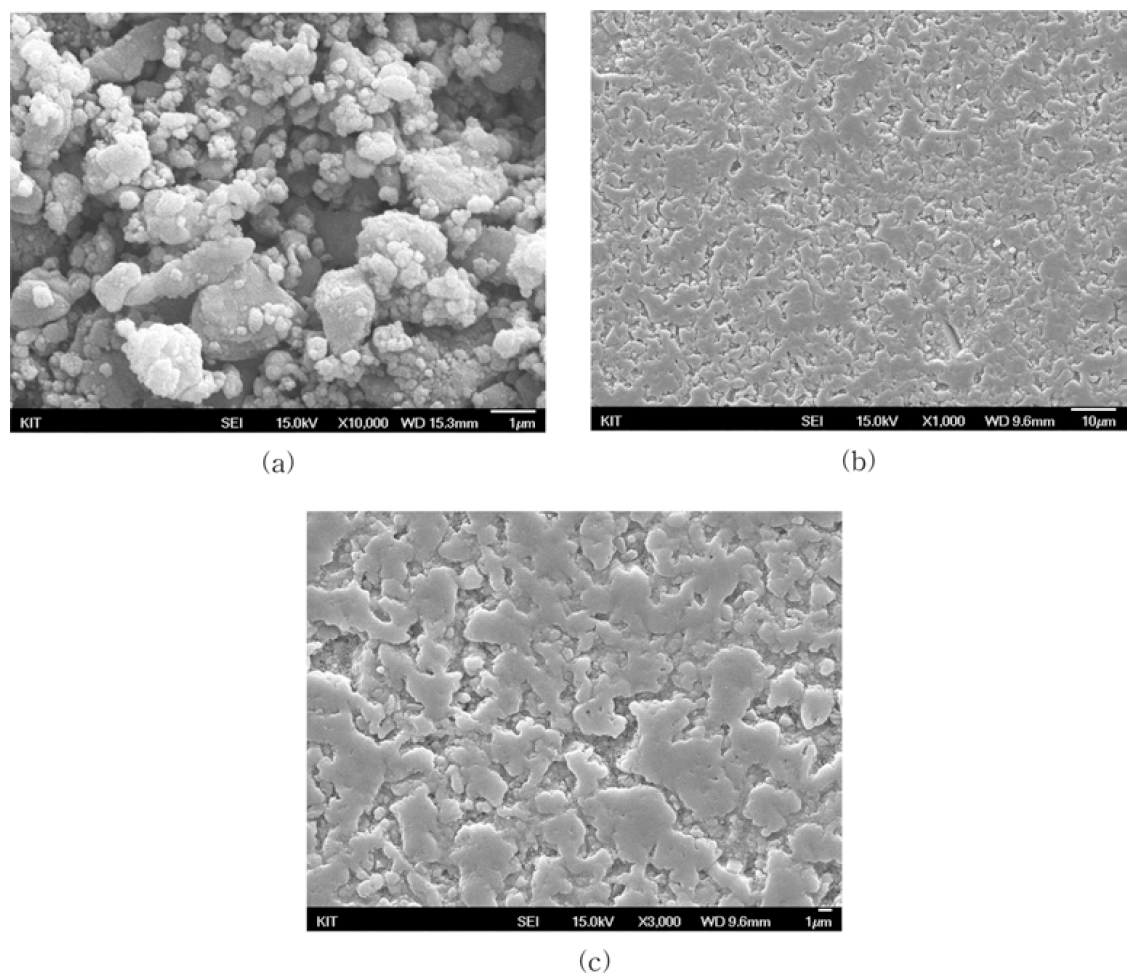
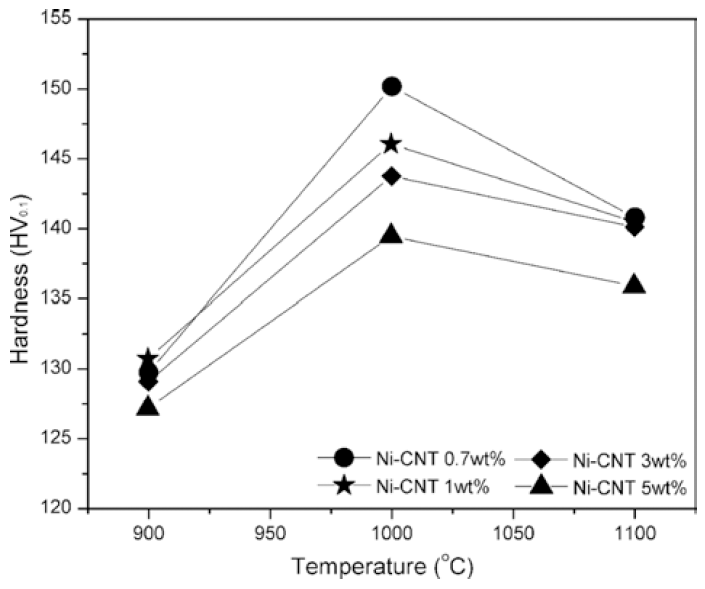
- Fabrication and Mechanical Characteristics of Bulk Nickel/Carbon Nanotube Nanocomposites via the Electrical Explosion of Wire in Liquid and Spark Plasma Sintering Method
- Thuyet-Nguyen Minh, Hai-Nguyen Hong, Won Joo Kim, Ho Yoon Kim, Jin-Chun Kim
- J Korean Powder Metall Inst. 2016;23(3):213-220. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.213
- 1,278 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, bulk nickel-carbon nanotube (CNT) nanocomposites are synthesized by a novel method which includes a combination of ultrasonication, electrical explosion of wire in liquid and spark plasma sintering. The mechanical characteristics of the bulk Ni-CNT composites synthesized with CNT contents of 0.7, 1, 3 and 5 wt.% are investigated. X-ray diffraction, optical microscopy and field emission scanning electron microscopy techniques are used to observe the different phases, morphologies and structures of the composite powders as well as the sintered samples. The obtained results reveal that the as-synthesized composite exhibits substantial enhancement in the microhardness and values more than 140 HV are observed. However an empirical reinforcement limit of 3 wt.% is determined for the CNT content, beyond which, there is no significant improvement in the mechanical properties.
-
Citations
Citations to this article as recorded by- Fabrication of nanocomposites by electric explosion of stainless steel capillaries filled with carbon nanotubes
Tao Jiang, Zhongyu Hou
Applied Surface Science.2020; 513: 145824. CrossRef - Effect of a nano-sized TiC particle addition on the flow-assisted corrosion resistance of SA 106B carbon steel
Jin-Ju Park, Eun-Kwang Park, Gyoung-Ja Lee, Chang-Kyu Rhee, Min-Ku Lee
Applied Surface Science.2017; 415: 143. CrossRef
- Fabrication of nanocomposites by electric explosion of stainless steel capillaries filled with carbon nanotubes
- [Korean]
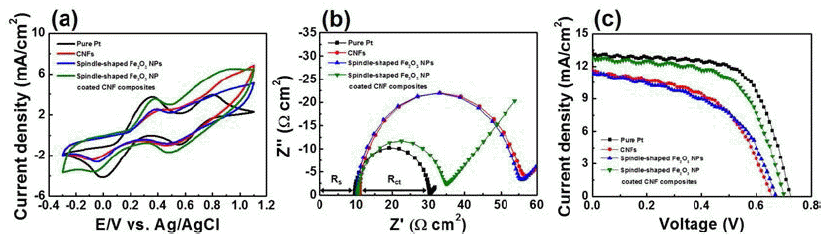
- Spindle-shaped Fe2O3 Nanoparticle Coated Carbon Nanofiber Composites for Low-cost Dye-sensitized Solar Cells
- Dong-Hyeun Oh, HyeLan An, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(2):95-101. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.95
- 1,044 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Carbon nanofiber (CNF) composites coated with spindle-shaped Fe2O3 nanoparticles (NPs) are fabricated by a combination of an electrospinning method and a hydrothermal method, and their morphological, structural, and chemical properties are measured by field-emission scanning electron microscopy, transmission electron microscopy, Xray diffraction, and X-ray photoelectron spectroscopy. For comparison, CNFs and spindle-shaped Fe2O3 NPs are prepared by either an electrospinning method or a hydrothermal method, respectively. Dye-sensitized solar cells (DSSCs) fabricated with the composites exhibit enhanced open circuit voltage (0.70 V), short-circuit current density (12.82 mA/cm2), fill factor (61.30%), and power conversion efficiency (5.52%) compared to those of the CNFs (0.66 V, 11.61 mA/cm2, 51.96%, and 3.97%) and spindle-shaped Fe2O3 NPs (0.67 V, 11.45 mA/cm2, 50.17%, and 3.86%). This performance improvement can be attributed to a synergistic effect of a superb catalytic reaction of spindle-shaped Fe2O3 NPs and efficient charge transfer relative to the one-dimensional nanostructure of the CNFs. Therefore, spindle-shaped Fe2O3-NPcoated CNF composites may be proposed as a potential alternative material for low-cost counter electrodes in DSSCs.
-
Citations
Citations to this article as recorded by- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
Dong-Hyeun Oh, Bon-Ryul Koo, Yu-Jin Lee, HyeLan An, Hyo-Jin Ahn
Korean Journal of Materials Research.2016; 26(11): 649. CrossRef
- Ni Nanoparticles-Graphitic Carbon Nanofiber Composites for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
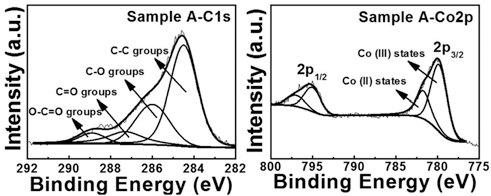
- Synthesis of Perforated Polygonal Cobalt Oxides using a Carbon Nanofiber Template
- Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(5):350-355. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.350
- 831 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Perforated polygonal cobalt oxide (CO3O4) is synthesized using electrospinning and a hydrothermal method followed by the removal of a carbon nanofiber (CNF) template. To investigate their formation mechanism, thermogravimetric analysis, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and Xray photoelectron spectroscopy are examined. To obtain the optimum condition of perforated polygonal CO3O4, we prepare three different weight ratios of the Co precursor and the CNF template: sample A (Co precursor:CNF template- 10:1), sample B (Co precursor:CNF template-3.2:1), and sample C (Co precursor:CNF template-2:1). Among them, sample A exhibits the perforated polygonal CO3O4 with a thin carbon layer (5.7-6.2 nm) owing to the removal of CNF template. However, sample B and sample C synthesized perforated round CO3O4 and destroyed CO3O4 powders, respectively, due to a decreased amount of Co precursor. The increased amount of the CNF template prevents the formation of polygonal CO3O4. For sample A, the optimized weight ratio of the Co precursor and CNF template may be related to the successful formation of perforated polygonal CO3O4. Thus, perforated polygonal CO3O4 can be applied to electrode materials of energy storage devices such as lithium ion batteries, supercapacitors, and fuel cells.
-
Citations
Citations to this article as recorded by- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef - Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef
- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
- [Korean]
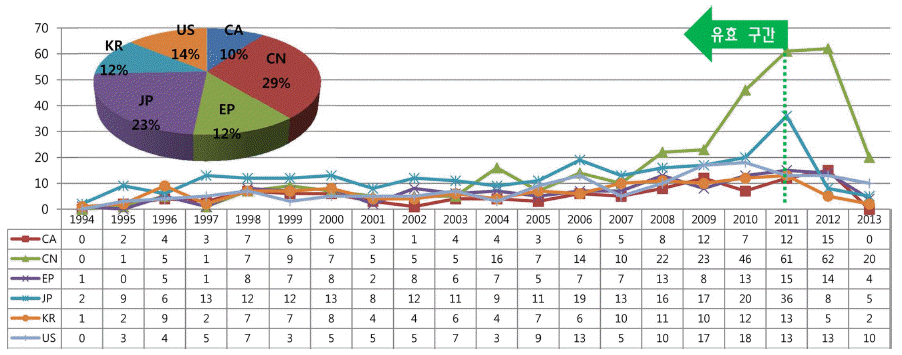
- Technology Trend Analysis of CO2 Capture and Storage by Patent Information
- Su-Jin Lee, Yun-Seock Lee, Jeong-Gu Lee, Soon-Jik Hong, Joong-Beom Lee
- J Korean Powder Metall Inst. 2015;22(4):289-297. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.289
- 702 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF As recognized by all scientific and industrial groups, carbon dioxide(CO2) capture and storage(CCS) could play an important role in reducing greenhouse gas emissions. Especially carbon capture technology by dry sorbent is considered as a most energy-efficient method among the existing CCS technologies. Patent analysis has been considered to be a necessary step for identifying technological trend and planning technology strategies. This paper is aimed at identifying evolving technology trend and key indicators of dry sorbent from the objective information of patents. And technology map of key patents is also presented. In this study the patents applied in korea, japan, china, canada, US, EU from 1993 to 2013 are analyzed. The result of patent analysis could be used for R&D and policy making of domestic CCS industry.
- [Korean]
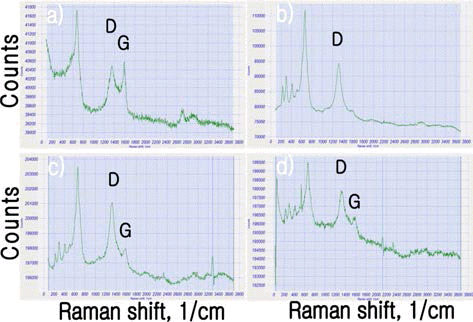
- Powder Sintering Characteristics of Carbon Nanotubes Reinforced SKD11 Tool Steel Sintered by Spark Plasma Sintering
- Je-Se Moon, Sung-Sil Jung, Dae-Yeol Lee, Young-Keun Jeong, Myung Chang Kang, Chun-Dal Park, Kook-Tae Youn
- J Korean Powder Metall Inst. 2015;22(3):157-162. Published online June 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.3.157
- 903 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF SKD11 (ASTM D2) tool steel is a versatile high-carbon, high-chromium, air-hardening tool steel that is characterized by a relatively high attainable hardness and numerous, large, chromium rich alloy carbide in the microstructure. SKD11 tool steel provides an effective combination of wear resistance and toughness, tool performance, price, and a wide variety of product forms. Adding of CNTs increased the performance of mechanical properties more. 1, 3 vol% CNTs was dispersed in SKD11 matrix by mechanical alloying. SKD11 carbon nanocomposite powder was sintered by spark plasma sintering process. FE-SEM, HR-TEM and Raman analysis were carried out for the SKD11 carbon nanocomposites.
-
Citations
Citations to this article as recorded by- Study on Effects of Mold Temperature on the Injection Molded Article
J.-H. Han, Y.-C. Kim
Archives of Metallurgy and Materials.2017; 62(2): 1271. CrossRef
- Study on Effects of Mold Temperature on the Injection Molded Article
- [Korean]
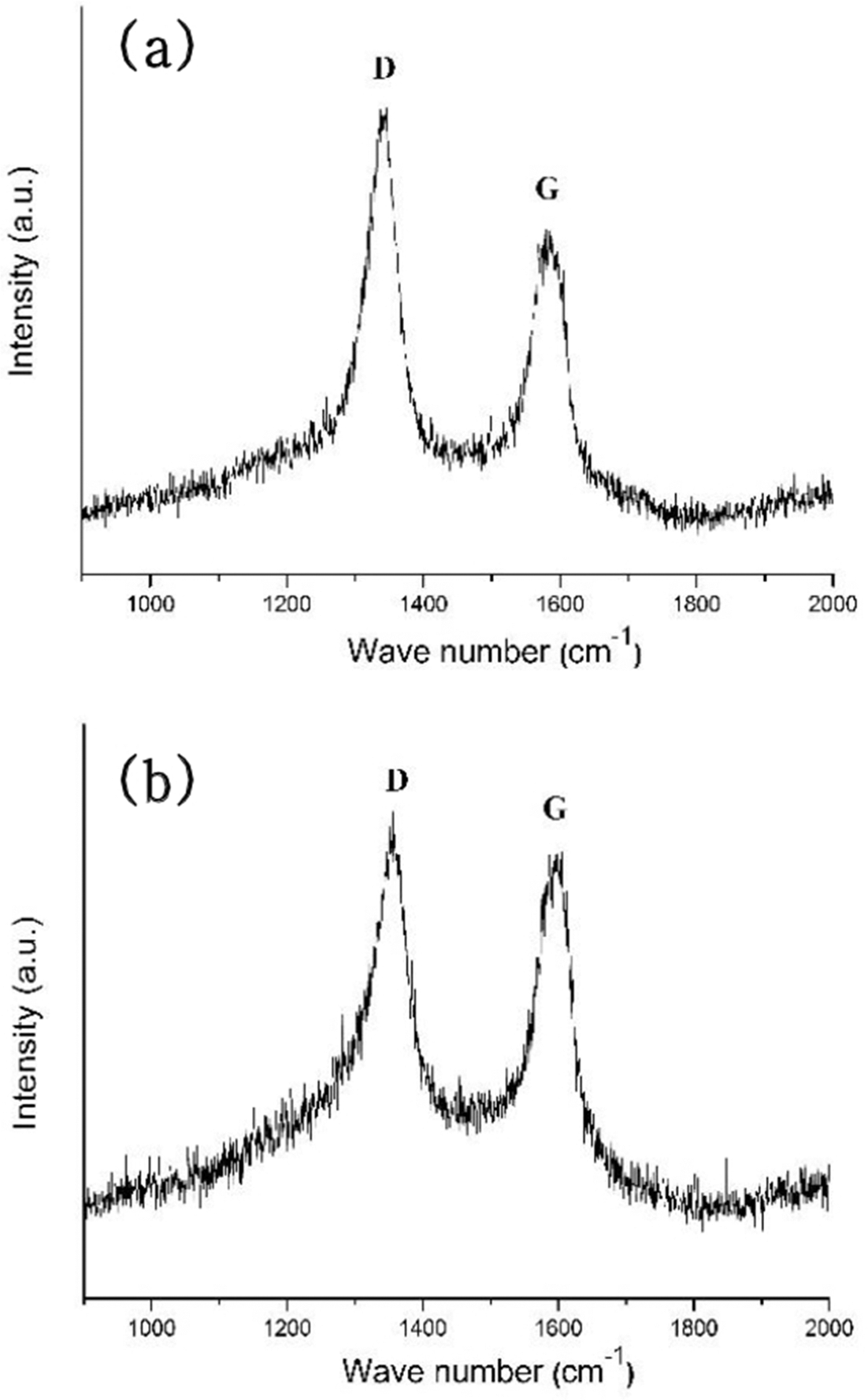
- Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
- Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(2):122-128. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.122
- 787 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, titanium(Ti) meshes and porous bodies are employed to synthesize carbon nanotubes(CNTs) using methane(CH4) gas and camphene solution, respectively, by chemical vapor deposition. Camphene is impregnated into Ti porous bodies prior to heating in a furnace. Various microscopic and spectroscopic techniques are utilized to analyze CNTs. It is found that CNTs are more densely and homogeneously populated on the camphene impregnated Ti-porous bodies as compared to CNTs synthesized with methane on Ti-porous bodies. It is elucidated that, when synthesized with methane, few CNTs are formed inside of Ti porous bodies due to methane supply limited by internal structures of Ti porous bodies. Ti-meshes and porous bodies are found to be multi-walled with high degree of structural disorders. These CNTs are expected to be utilized as catalyst supports in catalytic filters and purification systems.
-
Citations
Citations to this article as recorded by- Recent progress in additive manufacturing of porous titanium: From design to applications
Haoxin Song, Chen Wang, Wenzheng Yu, Mingsen Zhang, Jinqiang Shao, Hanwen Liang, Tingting Wu, Xiaoxiao Dong
Journal of Alloys and Compounds.2025; 1026: 180451. CrossRef - Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef
- Recent progress in additive manufacturing of porous titanium: From design to applications
- [Korean]
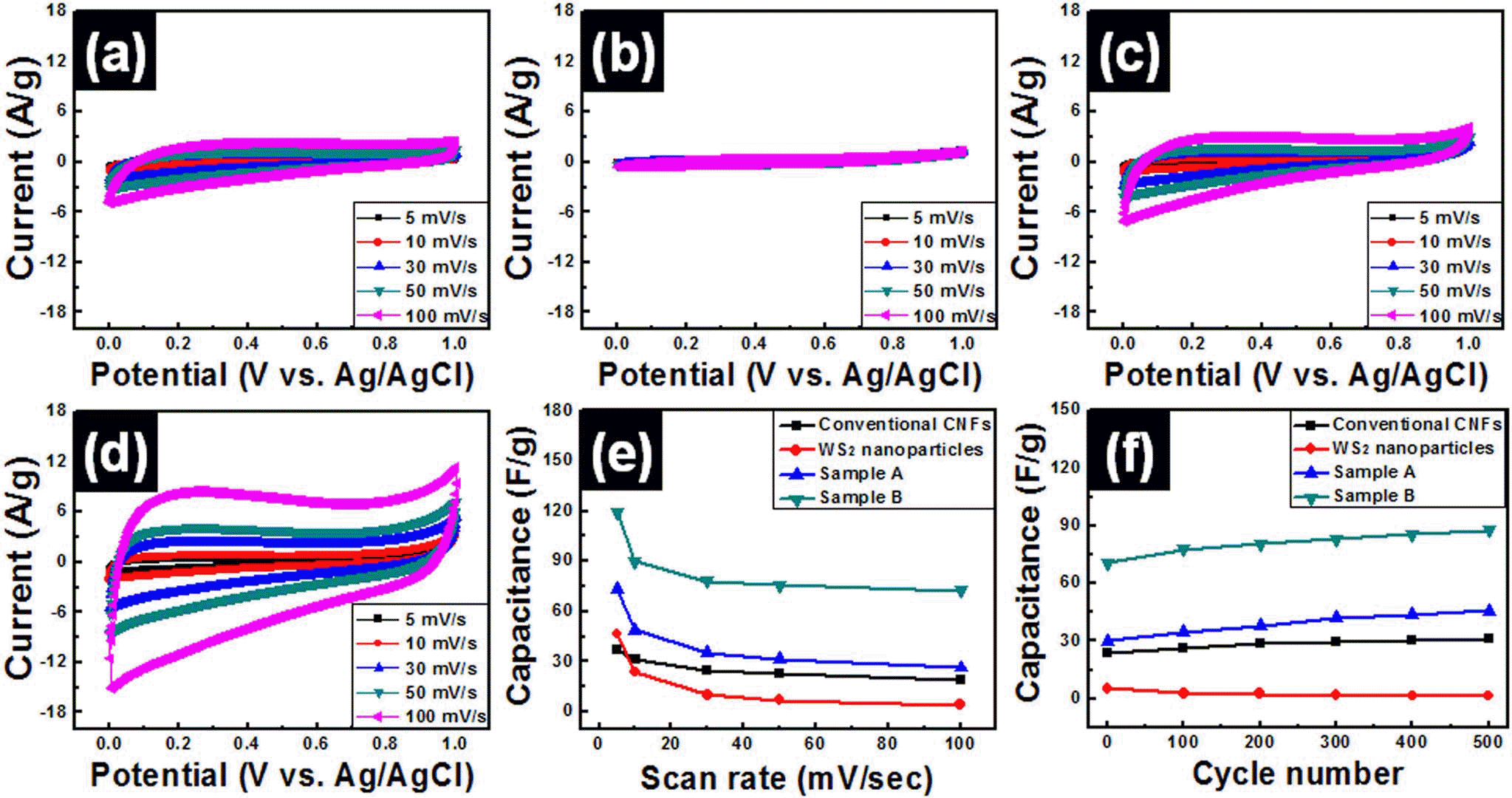
- Fabrication of WS2-W-WC Embedded Carbon Nanofiber Composites for Supercapacitors
- Yu-Jin Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(2):116-121. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.116
- 848 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF WS2-W-WC embedded carbon nanofiber composites were fabricated by using electrospinning method for use in high-performance supercapacitors. In order to obtain optimum electrochemical properties for supercapacitors, WS2 nanoparticles were used as precursors and the amounts of WS2 precursors were controlled to 4 wt% (sample A) and 8 wt% (sample B). The morphological, structural, and chemical properties of all samples were investigated by means of field emission photoelectron spectroscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. These results demonstrated that the embedded phases of samples A and B were changed from WS2 to WS2-W-WC through carbothermal reaction during carbonization process. In particular, sample B presented high specific capacitance (~119.7 F/g at 5 mV/s), good high-rate capacitance (~60.5%), and superb cycleability. The enhanced electrochemical properties of sample B were explained by the synergistic effect of the using 1-D structure supports, increase of specific surface area, and improved conductivity from formation of W and WC phases.
-
Citations
Citations to this article as recorded by- WS2 Nanoparticles Embedded in Carbon Nanofibers for a Pseudocapacitor
Ki-Wook Sung, Jung Soo Lee, Tae-Kum Lee, Hyo-Jin Ahn
Korean Journal of Materials Research.2021; 31(8): 458. CrossRef
- WS2 Nanoparticles Embedded in Carbon Nanofibers for a Pseudocapacitor
- [English]
- Powder Injection Molding of Translucent Alumina using Supercritical Fluid Debinding
- Hyung Soo Kim, Jong Min Byun, Myung Jin Suk, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(6):407-414. Published online December 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.6.407
- 1,032 View
- 1 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The powder injection molding process having advantages in manufacturing three-dimensional precision parts essentially requires a debinding process before sintering to remove the binders used for preparing feedstock. In this study, powder injection molding of translucent alumina was performed, and carbon dioxide (CO2) is used as a supercritical fluid that makes it possible to remove a large amount of binder, which is paraffin wax. The relationship between the optical property of translucent alumina and the debinding condition (temperature and pressure) of supercritical CO2 was investigated. As temperature and pressure increased, extraction rate of the binder showed rising tendency and average grain size after sintering process was relatively fine. On the other hand, optical transmittance was reduced. As a result, the debinding condition at 50° and 20 MPa that represents the lowest extraction rate, 8.19 × 10−3 m2/sec, corresponds to the largest grain size of 14.7 μm and the highest optical transmittance of 45.2%.
-
Citations
Citations to this article as recorded by- Supercritical CO₂ debinding: A promising method for binder removal in ceramic fabrication with potential applications in additive manufacturing – A review
Adolor David Aiyeki
Journal of the European Ceramic Society.2026; 46(5): 117998. CrossRef - Experimental and numerical analysis of effects of supercritical carbon dioxide debinding on Inconel 718 MIM components
Dugauguez Olivier, Agne Aboubabky, Jimenez-Morales Antonia, Torralba José Manuel, Barriere Thierry
Powder Technology.2019; 355: 57. CrossRef
- Supercritical CO₂ debinding: A promising method for binder removal in ceramic fabrication with potential applications in additive manufacturing – A review
- [Korean]
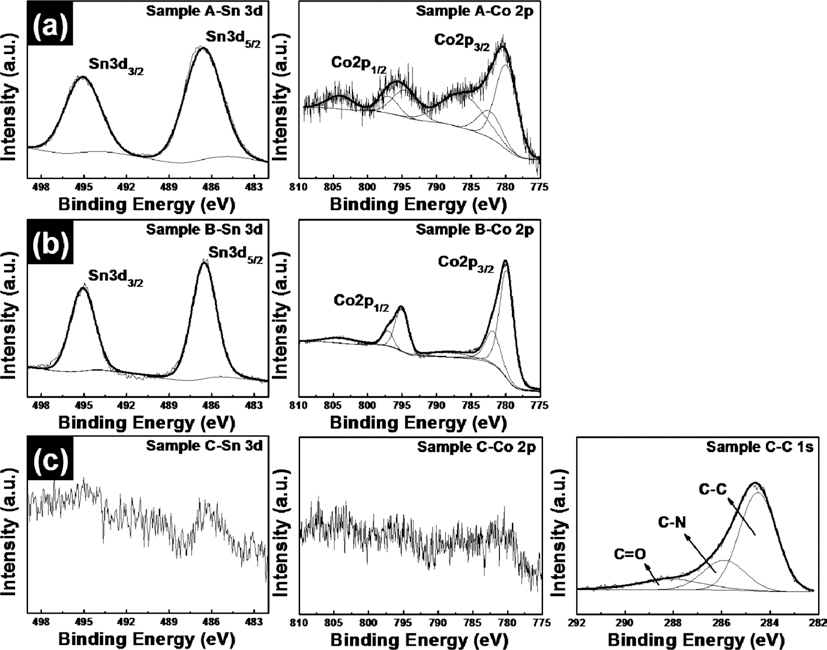
- Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
- Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(5):360-365. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.360
- 730 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF SnO2-CoO/carbon-coated CoO core/shell nanowire composites were synthesized by using electrospinning and hydrothermal methods. In order to obtain SnO2-CoO/carbon-coated CoO core/shell nanowire composites, SnO2-Co3O4 nanowire composites and SnO2-Co3O4/polygonal Co3O4 core/shell nanowire composites are also synthesized. To demonstrate their structural, chemical bonding, and morphological properties, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. These results indicated that the morphologies and structures of the samples were changed from SnO2-Co3O4 nanowires having cylindrical structures to SnO2-Co3O4/Co3O4 core/shell nanowires having polygonal structures after a hydrothermal process. At last, SnO2-CoO/carbon-coated CoO core/shell nanowire composites having irregular and high surface area are formed after carbon coating using a polypyrrole (PPy). Also, there occur phases transformation of cobalt phases from Co3O4 to CoO during carbon coating using a PPy under a argon atmosphere.
-
Citations
Citations to this article as recorded by- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
혜란 안, 혜린 강, 효정 선, 지호 한, 효진 안
Korean Journal of Materials Research.2015; 25(12): 672~677. CrossRef - Synthesis of Perforated Polygonal Cobalt Oxides usinga Carbon Nanofiber Template
Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2015; 22(5): 350. CrossRef
- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
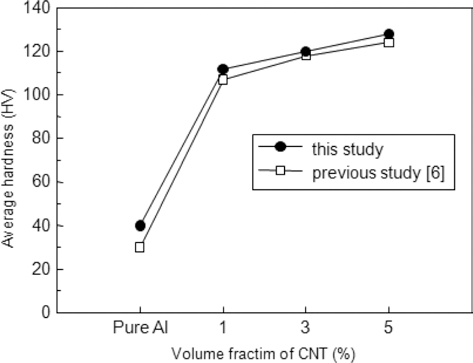
- Microstructure and Mechanical Properties of CNT/Al Composite Fabricated by a Powder-in-Sheath Rolling Method utilizing Copper Tube as a Sheath
- Seong-Hee Lee
- J Korean Powder Metall Inst. 2014;21(5):343-348. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.343
- 682 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF A powder-in-sheath rolling (PSR) process utilizing a copper alloy tube was applied to a fabrication of a multi-walled carbon nanotube (CNT) reinforced aluminum matrix composite. A copper tube with an outer diameter of 30 mm and a wall thickness of 2 mm was used as a sheath material. A mixture of pure aluminum powders and CNTs with the volume contents of 1, 3, 5 vol% was filled in the tube by tap filling and then processed to 93.3% height reduction by a rolling mill. The relative density of the CNT/Al composite fabricated by the PSR decreased slightly with increasing of CNTs content, but showed high value more than 98%. The average hardness of the 5%CNT/Al composite increased more than 3 times, compared to that of unreinforced pure Al powder compaction. The hardness of the CNT/Al composites was some higher than that of the composites fabricated by PSR using SUS304 tube. Therefore, it is concluded that the type of tube affects largely on the mechanical properties of the CNT/Al composites in the PSR process.
- [Korean]
- A Study of the Sintering Behavior of Boron Carbide using In-situ High Temperature Dilatometer
- Hyukjae Lee, Bum-Sup Kim, Tai-Joo Chung
- J Korean Powder Metall Inst. 2014;21(2):102-107. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.102

- 846 View
- 4 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF A high temperature dilatometer attached to a graphite furnace is built and used to study the sintering behavior of B4C. Pristine and carbon doped B4C compacts are sintered at various soaking temperatures and their shrinkage profiles are detected simultaneously using the dilatometer. Carbon additions enhance the sinterability of B4C with sintering to more than 97% of the theoretical density, while pristine B4C compacts could not be sintered above 91% due to particle coarsening. The shrinkage profiles of B4C reveal that the effect of carbon on the sinterability of B4C can be seen mostly below 1950°C. The high temperature dilatometer delivers very useful information which is impossible to obtain with conventional furnaces.
-
Citations
Citations to this article as recorded by- Application of rate-controlled sintering into the study of sintering behavior of boron carbide
Hyukjae Lee
Journal of the Korean Crystal Growth and Crystal Technology.2015; 25(1): 6. CrossRef
- Application of rate-controlled sintering into the study of sintering behavior of boron carbide
- [Korean]
- Fabrication and Evaluation of Carbon Nanotube Reinforced Al Matrix Composite by a Powder-in-sheath Rolling Method
- Seong-Hee Lee, Dongmin Hong
- J Korean Powder Metall Inst. 2014;21(1):50-54. Published online February 1, 2014
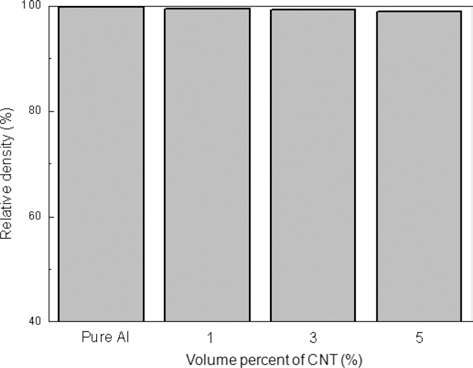
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.50
- 922 View
- 1 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
PDF A powder-in-sheath rolling method was applied to a fabrication of a carbon nano tube (CNT) reinforced aluminum composite. A STS304 tube with an outer diameter of 34 mm and a wall thickness of 2 mm was used as a sheath material. A mixture of pure aluminum powders and CNTs with the volume contents of 1, 3, 5 vol was filled in the tube by tap filling and then processed to 73.5% height reduction by a rolling mill. The relative density of the CNT/ Al composite fabricated by the powder-in-sheath rolling decreased slightly with increasing of CNTs content, but exhibited high value more than 98. The grain size of the aluminum matrix was largely decreased with addition of CNTs; it decreased from 24 μm to 0.9 μm by the addition of only 1 volCNT. The average hardness of the composites increased by approximately 3 times with the addition of CNTs, comparing to that of unreinforced pure aluminum. It is concluded that the powder-in-sheath rolling method is an effective process for fabrication of CNT reinforced Al matrix composites.
-
Citations
Citations to this article as recorded by- Torsion Property of the Structure Bonded Aluminum Foam Due to Impact
G.W. Hwang, J.U. Cho
Archives of Metallurgy and Materials.2017; 62(2): 1353. CrossRef - A Fatigue Fracture Study on TDCB Aluminum Foam Specimen of Type Mode III Bonded with Adhesive
J.H. Lee, J.U. Cho
Archives of Metallurgy and Materials.2017; 62(2): 1359. CrossRef - Experimental Study On Fracture Property Of Double Cantilever Beam Specimen With Aluminum Foam
Y.C. Kim, H.K. Choi, J.U. Cho
Archives of Metallurgy and Materials.2015; 60(2): 1151. CrossRef - Experimental Study On Fracture Property Of Tapered Double Cantilever Beam Specimen With Aluminum Foam
Y.C. Kim, S.S. Kim, J.U. Cho
Archives of Metallurgy and Materials.2015; 60(2): 1459. CrossRef - Microstructure and Mechanical Properties of CNT/Al Composite Fabricated by a Powder-in-Sheath Rolling Method utilizing Copper Tube as a Sheath
Seong-Hee Lee
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 343. CrossRef
- Torsion Property of the Structure Bonded Aluminum Foam Due to Impact
- [Korean]
- Study on Surface Modification of Ti Substrate to Improve the Dispersion of Catalytic Metals on Synthesis of Carbon Nanotubes
- Seoung Yeol Kwak, Ho Gyu Kim, Jong Min Byun, Ju Hyuk Park, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(1):28-33. Published online February 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.1.28
- 894 View
- 0 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF This paper describes the surface modification effect of a Ti substrate for improved dispersibility of the catalytic metal. Etching of a pure titanium substrate was conducted in 50% H2SO4, 50°C for 1 h-12 h to observe the surface roughness as a function of the etching time. At 1 h, the grain boundaries were obvious and the crystal grains were distinguishable. The grain surface showed micro-porosities owing to the formation of micro-pits less than 1 μm in diameter. The depths of the grain boundary and micro-pits appear to increase with etching time. After synthesizing the catalytic metal and growing the carbon nano tube (CNT) on Ti substrate with varying surface roughness, the distribution trends of the catalytic metal and grown CNT on Ti substrate are discussed from a micro-structural perspective.
-
Citations
Citations to this article as recorded by- Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef - Spontaneous Formation of Titanium Nitride on the Surface of a Ti Rod Induced by Electro-Discharge-Heat-Treatment in an N2 Atmosphere
W.H. Lee, Y.H. Yoon, Y.H. Kim, Y.K. Lee, J.Y. Kim, S.Y. Chang
Archives of Metallurgy and Materials.2017; 62(2): 1281. CrossRef - Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
Journal of Korean Powder Metallurgy Institute.2015; 22(2): 122. CrossRef
- Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
TOP
 KPMI
KPMI


 First
First Prev
Prev


